Introduction
Cytostatics, comprising synthetic or natural compounds, are the cornerstone of cancer treatment. Their mode of action varies depending on the specific agent, primarily involving the inhibition of cell division and induction of apoptosis. Many anti-cancer drugs require high doses to achieve efficacy [1]. However, conventional chemotherapy indiscriminately attacks both cancerous and normal cells, leading to tissue and organ damage. Consequently, there is growing interest in natural remedies sourced from plants, microorganisms, and marine organisms [2, 3], which currently represent significant anti-cancer modalities [4]. Characterised by minimal side effects compared to conventional chemotherapy, these natural compounds additionally offer protective benefits to unaffected organs and tissues [3]. For instance, honey and royal jelly have demonstrated protective properties against cisplatin- induced nephrotoxicity, a common side effect of this anti-cancer agent [2, 3].
The historical use of natural products dates back to ancient civilisations, with their therapeutic efficacy acknowledged in Mesopotamia as early as 2600 BC [5]. In 1991, the mummified body of a man who probably lived around 5300 years ago was discovered in Tyrol [6–10]. Among the items found with him was a fragment of Fomitopsis betulina (previously known as Piptoporus betulinus), suggesting that the medicinal properties of mushrooms might have been utilised even then [7–9]. Fomitopsis betulina, occurring in the Northern Hemisphere, grows exclusively on birch trees, both dead and alive, causing brown rot of the wood. The fruiting bodies of this mushroom are annual, ranging in colour from white to grey-brown, and can appear individually or in groups, reaching sizes of 5–20 cm in width and 2–6 cm in thickness [10]. This mushroom is classified as inedible, although some consider young fruiting bodies to be edible [9, 10]. Fomitopsis betulina emits a strong, pleasant, slightly bitter aroma, and has an astringent-bitter taste [9]. The potential activity of Fomitopsis betulina has been confirmed by scientific research. This mushroom possesses many pharmacological properties, including antiseptic, anti-inflammatory, antibacterial, anticancer, antiparasitic, analgesic, and immune-boosting effects [9, 10]. Biological activity is exhibited by extracts obtained from the mushroom and compounds isolated from it [10]. The extract can be obtained from both the fruiting bodies and the mycelium [6]. Extraction methods vary in different research studies. To date, water, ether, methanol, chloroform, ethanol, and ethanol-acetic extracts have been tested, showing varying degrees of biological activity [10]. Research on Fomitopsis betulina in the form of extracts relates to its traditional uses [7]. Tea made from the mushroom was popular in Baltic countries due to its calming effects and nutritional values. A decoction prepared from Fomitopsis betulina was used as a remedy for gastrointestinal disorders, as an antiparasitic, and as an immune booster. It was also consumed for various types of cancer [9]. Extracts from the mushroom often show greater bioactivity than isolated compounds at the same dose, related to the phenomenon of synergy between the individual compounds in the extract [7].
The reclassification of Fomitopsis betulina and thus its nomenclature has not been fully adopted. This is evident from the fact that some scientists still use the previous terminology. The name Piptoporus betulinus can still be encountered in papers published after 2016. Some researchers use both names in their papers, but give the obsolete name, which remains a scientific synonym, as the main one. This is probably the result of being accustomed to the old terminology or the new one not being accepted in the scientific world. This leads to difficulties and issues when searching for publications concerning studies on this fungus. People without a background in mycology may be confused and believe 2 different species of fungi are involved. The warty birch (Betula pendula Roth) is valued worldwide for its medicinal properties. It has a prominent place in folklore and has also been a source of various materials for generations. One of them is Fomitopsis betu-lina [11]. This fungus, parasitising on birch, decomposes its wood using the enzymes it produces, thereby acquiring the nutrients it needs to survive [12]. The composition of this fungus includes the same substances present in birch bark, which exhibit biological potential [11]. These include betulin and betulinic acid, which are classified as triterpenes. It is this group, especially the lantosan derivatives, that is primarily responsible for the medicinal properties of the fungus [11, 13].
In summary, the potential anticancer properties of Fomitopsis betulina have garnered attention in recent studies. The limited research on Fomitopsis betulina underscores its attractiveness, particularly based on preliminary findings from basic in vitro studies. It is crucial to note that the incomplete exploration of this fungus raises questions about its full range of therapeutic benefits and potential side effects. Therefore, further targeted research is imperative to comprehensively understand the cytotoxic effects of Fomitopsis betulina on both normal and cancer cells. The scarcity of information on this fungus emphasises the need for more in-depth investigations to unveil its true potential in the realm of oncology. This paper aims to provide a systematic review of studies pertaining to Fomitopsis betulina (Bull.) B.K Cui, M.L. Han & Y.C. Dai, specifically elucidating its cytotoxic effects on both normal and cancer cells.
Material and methods
Search strategy and selection criteria
Three databases – PubMed, Google Scholar, and Web of Sciences – were queried for articles up to July 2024. Initial searches utilised database-specific MeSH terms including “cancer”, “in vitro”, “cytotoxicity”, “therapy”, “systematic review”, and “meta-analysis” with adjustments made to accommodate each database’s vocabulary map. Various categories of terms were explored, such as “cell cultures”, “cancer”, “cytotoxicity”, “cell viability”, “cancer cells”, “MTT assay”, “LDH assay”, “apoptotic cells”, and “in vitro study”. The search criteria incorporated terms like (cancer* OR cancer cell line* OR cell culture* or in vitro study*) AND (cytotoxicity OR cell viability) AND (MTT assay OR LDH assay OR apoptotic cells). To ensure comprehensive coverage of the literature, synonyms for Fomitopsis betulina, such as Piptoporus betulinus, were also included in the search. The search terms used were adapted for each database’s specific vocabulary. The literature search was independently performed by 2 authors (PN and BW).
Article inclusion was contingent upon the utilisation of both cancer and normal cell cultures in the study facilitating subsequent in vivo and preclinical investigations. Criteria for inclusion encompassed factors such as the determination of the IC50 value (the concentration at which cell viability diminishes by 50% compared to untreated controls), the viability of normal and cancer cell cultures, and the presentation of research findings through qualitative, quantitative, or mixed methods. The selected studies were confined to in vitro investigations evaluating the impact of Fomitopsis betulina extracts on cancer cell viability, inevitably implicating normal cell viability as well. Articles were filtered using the PRISMA format, with editorials, reviews, and expert opinions excluded [14]. No language restrictions were imposed, and only full-text research papers from peer-reviewed journals were considered. Any discrepancies regarding article inclusion or exclusion were resolved through consensus among all authors.
Data abstraction
Two independent authors (PN and BW) conducted data extraction. Information gathered included the publication year, study design, cytotoxicity assay (e.g. MTT assay, LDH assay, apoptotic and necrotic cells), cell lines, and cancer cell line types, as well as normal cell types.
Quality assessment of included articles
The quality of the included studies was assessed using the PRISMA guidelines [14]. Each study was evaluated based on a set of criteria, including the presence of a control group, the use of appropriate cytotoxicity assays, and the clarity of the reported outcomes. Each category item was assessed as “yes”, “no”, or “can’t say”. Studies that met more criteria were considered of higher quality and were denoted by more stars (*) in the assessment tables. Quality assessment was independently performed by 2 authors (PN and BW).
Two authors (PN and BW) independently conducted the quality assessment. Any discrepancies in the evaluation were resolved through discussion to reach a consensus. No studies were excluded based on quality assessment because they all met acceptable quality criteria. The criteria used for assessment included the following:
Study design and methodology: whether the study design was appropriate for the research question;
Control group: presence of a control group, particularly normal cell cultures alongside cancer cell cultures;
Cytotoxicity assays: use of validated and reliable assays (e.g. MTT assay, LDH assay) to measure cytotoxic effects;
Outcome reporting: clarity and comprehensiveness in reporting results, including IC50 values and statistical analyses.
The quality assessment results are presented in Table 1. This table summarises the assessment of each study and provides an overview of their respective strengths and weaknesses based on the predefined criteria. By maintaining a rigorous quality assessment process, this systematic review ensures that the findings are based on reliable and well-conducted studies, thereby enhancing the validity and credibility of the conclusions drawn.
Table 1
Quality assessment of included studies (overall quality – an assessment indicator based on the number of “Yes” responses)
| Study | Study design and methodology | Control group | Cytotoxicity assays | Outcome reporting | Overall quality |
|---|---|---|---|---|---|
| Sułkowska-Ziaja [6] | Yes | Yes | Yes | Yes | **** |
| Czerwonka [15] | Yes | Yes | Yes | Can’t say | *** |
| Sofrenić [13] | Yes | Yes | Yes | Yes | **** |
| Bożek [16] | Yes | Yes | Yes | Can’t say | *** |
| Kozarski [8] | Yes | Yes | Yes | Yes | **** |
Results
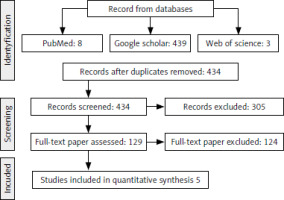
The literature search across the 3 databases yielded a total of 450 articles. Sixteen abstracts were excluded due to duplication, and 305 articles were deemed irrelevant, being reviews, expert opinions/editorials, or unrelated to the subject matter under review. Of the remaining 129 articles, only 5 met the criteria for inclusion. The 124 articles rejected did not meet significance criteria because they were either not peer-reviewed, lacked a control group (normal cell culture), or were deemed irrelevant (Fig. 1). Of the full-text articles, 64 articles were excluded because they did not meet the inclusion criteria, i.e. they did not include both healthy and cancer cell lines. In 42 studies, the results were presented inadequately, often as graphical data only, without precise numerical values, such as IC50, necessary for inclusion. In addition, 18 articles did not provide sufficient details regarding the specific cytotoxicity tests used, because they did not specify the methods used to assess cytotoxic effects.
The included studies are presented in Table 1. All studies identified in this review were quantitative. The studies were mainly conducted to evaluate the potential cytotoxic effect of Fomitopsis betulina extracts in cancer cell cultures. The research included, among others, a comparison of the impact of the tested Fomitopsis betulina extracts on the viability of normal cells. So far, alcohol (both ethanol and methanol) and aqueous extracts have been tested. At the same time, selected single compounds, mainly from the group of terpenes, were also tested (Table 2).
Table 2
List of papers included in the systematic review
| No. | Author | Title | Study design | Outcome |
|---|---|---|---|---|
| 1. | Sułkowska-Ziaja [6] | Chemical composition and biological activity of extracts from fruiting bodies and mycelial cultures of Fomitopsis betulina | DU145 PNT-2 WM795 A375 BJ | Methanol extract of fruiting bodies and methanolic extract of the mycelial chanterelles of Fomitopsis betulina |
| 2. | Czerwonka [15] | Antitumour effect of glucooligosaccharides obtained via hydrolysis of α-(1→3)-glucan from Fomitopsis betulina | HT-29 CCD841- CoTr LS180 SW620 SW948 | α-(1 → 3)-glucooligosaccharides obtained by acid hydrolysis of α-(1 → 3)-glucan from the cell wall of Fomitopsis betulin fruiting bodies |
| 3. | Sofrenić [16] | Cytotoxic triterpenoids and triterpene sugar esters from the medicinal mushroom Fomitopsis betulina | HL-60 A549 MRC-5 | CHCL3/CH3OH (2:1) extract of air-dried, sprinked fruit Fomitopsis betulina (200g) Triterpenes isolated |
| 4. | Bożek [16] | Effects of Piptoporus betulinus Ethanolic extract on the proliferation and viability of melanoma cells and models of their cell membranes | WM 115 A375 Hs27 | Ethanolic extract of Fomitopsis betulina |
| 5. | Kozarski [8] | Bioprospecting of selected species of polypore fungi from the Western Balkans | HeLa K562 MDA-MB-453 MRC-5 BEAS-2B | Methanol extract of Fomitopsis betulina |
Cytotoxicity of Fomitopsis betulina extracts and compounds
The cytotoxicity of various Fomitopsis betulina extracts and their isolated compounds was assessed against multiple cell lines, including both normal and cancerous cells. The results provide insights into the selective toxicity and potential therapeutic uses of these extracts and compounds (Table 3).
Table 3
Cytotoxicity of the most relevant compounds and Fomitopsis betulina extracts in in vitro tests along with the selectivity index assessment
A549 – lung carcinoma, BEAS-2B – normal human bronchial epithelium, BJ – normal fibroblasts, CCD 841 CoTr – normal colon cells, DU145 – prostate carcinoma, HeLa – cervical adenocarcinoma, HL-60 – acute promyelocytic leukaemia, Hs27 – normal skin fibroblasts, HT-29, SW948 – colorectal carcinoma, K562 – myelogenous leukaemia, LS180, MDA-MB-453 – breast metastatic carcinoma [6, 8, 13, 15, 16], MRC-5 – normal lung fibroblasts, PNT-2 – normal prostate cells, SI – selectivity index, SW620 – colorectal adenocarcinoma, WM115 – melanoma, WM795, A375
The extracts from the fruiting bodies of Fomitopsis betulina demonstrated varied cytotoxic effects across different cell lines. For normal cell lines, both BJ (normal fibroblasts) and PNT-2 (normal prostate cells) exhibited an IC50 value of 100 µg/ml. In contrast, the DU145 prostate carcinoma cell line showed a significantly lower IC50 value of 48.19 µg/ml, indicating higher sensitivity to the extract. The melanoma cell lines WM795 and A375 had IC50 values of 100 µg/ml and 56.88 µg/ml, respectively. The selectivity index (SI), which measures the ratio of IC50 values between normal and cancer cells, was calculated as 1.00 for BJ/WM795, 1.76 for BJ/A375, and 2.07 for PNT-2/DU145. These indices suggest that the extracts have moderate selectivity towards cancer cells over normal cells.
The extracts from the mycelial culture of Fomitopsis betulina displayed a similar pattern but with higher selectivity indices. For normal cells, both BJ and PNT-2 again showed IC50 values of 100 µg/ml. The DU145 prostate carcinoma cell line exhibited a much lower IC50 value of 16.11 µg/ml. For melanoma cell lines WM795 and A375, the IC50 values were 65.70 µg/ml and 43.02 µg/ml, respectively. The selectivity indices were 1.52 for BJ/WM795, 2.32 for BJ/A375, and a notably higher 6.21 for PNT-2/DU145, indicating a stronger selective cytotoxic effect of the mycelial extracts on cancer cells compared to the fruiting body extracts.
The glucooligosaccharides (GOS) obtained via hydrolysis of α-(1-3)-glucan were tested on colorectal cancer cell lines. The IC50 values for normal colon cells (CCD 841 CoTr) and cancer cell lines (HT-29, LS180, SW620, SW948) were 39.38 µg/ml, 17.06 µg/ml, 19.55 µg/ml, 14.92 µg/ml, and 18.30 µg/ml, respectively. The selectivity indices ranged 2.01–2.64, indicating that these GOS possess a reasonable degree of selectivity towards cancer cells.
Among the most relevant isolated compounds, 12α- hydroxy-3α-oxalyloxy-24-methylene-lanost-8-en-26-oic acid showed IC50 values of 109.18 µM for normal lung fibroblasts (MRC-5), 72.28 µM for acute promyelocytic leukaemia cells (HL-60), and 115.68 µM for lung carcinoma cells (A549). The selectivity indices were 1.51 for MRC-5/HL-60 and 0.94 for MRC-5/A549, suggesting limited selectivity. Other compounds, such as dehydropachymic acid and pachymic acid, demonstrated higher selectivity indices of up to 9.77, particularly for MRC-5/HL-60 and MRC-5/A549, indicating stronger cytotoxic effects on cancer cells.
The ethanol extract of Fomitopsis betulina was tested on skin fibroblasts (Hs27) and melanoma cell lines (A375, WM115). The IC50 values were 23.07 µl/ml for Hs27, 3.21 µl/ml for A375, and 10.10 µl/ml for WM115. The selectivity indices were 7.20 for Hs27/A375 and 2.29 for Hs27/WM115, indicating significant selectivity towards melanoma cells.
Methanol extracts were tested on various cell lines, including normal lung fibroblasts (MRC-5) and human bronchial epithelium cells (BEAS-2B), as well as cancer cell lines such as HeLa, K562, and MDA-MB-453. The IC50 values were around 40–43 µg/ml for normal cells and 40–42 µg/ml for cancer cells, resulting in selectivity indices close to 1.00, which suggests minimal selectivity.
Discussion
Fomitopsis betulina – dual role in forest ecosystems and economic impact on taxonomic reclassification
Fomitopsis betulina (Bull.) B.K Cui, M.L. Han & Y.C. Dai is associated with numerous scientific synonyms [17]. All such synonyms are catalogued in the Index Fungorum online database under the supervision of Centre for Agriculture and Bioscience International, which also provides information on fungus classifications updated in line with new phylogenetic studies [18]. Until 2016, this fungus was classified under the genus Piptoporus as Piptoporus betulinus (Bull.) [19]. Taxonomic and phylogenetic studies have revealed the phylogenetic heterogeneity of species belonging to Fomitopsis and Piptoporus, leading to the decision to reclassify Piptoporus betulinus under Fomitopsis [17]. Consequently, all names used are included in this review.
Therapeutic potential and bioactive compounds of Fomitopsis betulina – molecular mechanisms
Examples of compounds isolated from Fomitopsis betu-lina include betulinic acid, polysaccharides, and triterpenes [6]. Fomitopsis betulina exhibits various molecular mechanisms that may be responsible for its therapeutic effects, including anticancer, anti-inflammatory, and antioxidant properties [7, 16]. The anticancer mechanisms may result from influencing apoptosis, inhibiting proliferation, and affecting the nuclear factor (NF)-κB pathway. Compounds from Fomitopsis betulina, such as betulinic acid, can induce apoptosis in cancer cells through mitochondrial and caspase-dependent pathways, inhibit cancer cell proliferation, and have anti-inflammatory effects by inhibiting pro-inflammatory cytokines. Induction of apoptosis is often associated with the activation of p53 protein and an increase in pro-apoptotic proteins like Bax, as well as a decrease in anti-apoptotic proteins like Bcl-2 [20, 21]. These compounds can also arrest the cell cycle in the G1/G0 or G2/M phases, preventing cancer cell proliferation [20, 22]. In studies conducted on triterpenes, some compounds belonging to this group, isolated from Fomitopsis betulina, showed cytotoxic activity against the human Caucasian promyelocytic leukaemia cell lines (HL-60). Compared to the commercial anti-cancer drug cisplatin, this activity was lower. Selectivity of some compounds towards the normal human lung fibroblast cell line (MRC-5) was also demonstrated, which was higher in comparison with the positive control. This confirmed the results of the earlier triterpenoid tests on the HL-60 line, where the cytotoxic activity was comparable to fluorouracil. Against the carcinoma human lung cell line (A549), none of the isolated compounds showed any significant cytotoxic effects [13]. During in vitro studies, betulin has exhibited effective action against cervical adenocarcinoma (HeLa) and against the epidermal cancer cell lines (A431). On the other hand, carbamate derivatives of betulinic acid and betulin exhibit cytotoxic selectivity towards these cell lines by inducing apoptosis [11]. Tests of a methanol extract prepared from Fomitopsis betu-lina fruit bodies have shown a reduced betulin and betulinic acid content compared to a mycelium extract prepared in the same way [6].
Betulin and its derivatives show a broad range of biolo-gical activities. It is easily transformable into betulinic acid, which actively acts on cancer cells while showing no toxicity up to doses of 500 mg/kg body weight in mice [11]. In naked mice, it effectively inhibited the growth of tumours induced by injecting human malignant melanoma (Mel-2, Mel-1) [23]. It has been shown that betulinic acid, when used under hypoxia during conservative therapy of malignant tumours of human glioma cells (U251MG, U343MG), can improve the effects of the therapy [11]. Further studies on betulin safety are necessary. Studies conducted on fish fibroblast (BF-2) and mouse fibroblast (NIH/3T3) cell lines have shown a cytotoxic effect of this compound. This involved the sensitivity of cell mitochondria to the effects of betulin. CC50 values (50% cytotoxic concentration) for these lines were comparable to the IC50 results obtained from tests on tumour lines by other researchers. Later it was confirmed by tests with an ethanol solution of betulin isolated from Fomitopsis betulina. The extract reduced the viability of melanoma (A375), primary melanoma (WM115), and human fibroblast (Hs27) cell lines [16].
Triterpene derivatives with a lantostanoid structure also form promising anti-inflammatory drugs [20]. They have the ability to inhibit cyclooxygenase I and 3-α-hydroxysteroid dehydrogenase [6, 10]. They have been isolated from a methanol extract from Fomitopsis betulina fruit bodies. These include polyporic acid A and polyporic acid C, which were first isolated from this fungus in 1940 [10, 24]. In animal tests involving polyporic acid A, its anti-inflammatory effect was confirmed, but the tests also showed its anti-bacterial properties. It has a high activity against Mycobacterium sp. and can inhibit Escherichia coli [10].
The anti-inflammatory action is achieved by inhibiting pro-inflammatory cytokines and enzymes. Compounds isolated from Fomitopsis betulina can reduce the production of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, leading to decreased inflammation [20, 21]. Inhibiting the activity of pro-inflammatory enzymes such as COX-2 also contributes to the anti-inflammatory effect [25, 26]. Compounds from Fomitopsis betulina can act as antioxidants, neutralising free radicals and reducing oxidative stress. These compounds can increase the expression of antioxidant enzymes such as superoxide dismutase and catalase [25].
An analysis of the chemical composition of Fomitopsis betulina fruit bodies showed that they contain polysaccharides, located in the cell walls of the fungus. They can be classified into water-soluble β-glucans and base-soluble α-glucans. The latter, when brought into the carboxymethyl form, which is water-soluble, are characterised by a moderate cytotoxic activity in in vitro studies [10, 15]. A carboxylic derivative of α-(1 → 3)-D-glucan causes a reduced metabolism of the cervical adenocarcinoma line (HeLa). Another purified and isolated complex sugar, obtained from dried silkworm larvae (Bombyx batryticatus), induces apoptosis and stops the cell cycle of this cell line [15]. An α-(1 → 3)-GOS obtained via hydrolysis of an α-(1 → 3)-glucan from the Fomitopsis betulina fungus showed approximately twice as strong an effect on tumour cells as on normal colon cells [15]. β – D glucans also exhibit a biological activity. They stimulate the immune system, have an anti-bacterial and anti-oxidative effect, and inhibit the angiotensin-converting enzyme. (1 → 3), (1 → 6) – β – D – glucan isolated from Fomitopsis betulina fruit bodies at a concentration of 2000 µg/ml-1 reduced the viability of colon cancer cells (Caco-2 ) by 85%. Lower concentrations showed a very low cytotoxic activity on this cell line [27]. Studies conducted on oligosaccharides confirm their anti-cancer activity. They can cause the inhibition of the cell cycle and proliferation, and they can initiate the apoptosis process [15].
Polysaccharides enhance immune response by stimulating the activity of macrophages, natural killer (NK) cells, and T lymphocytes, leading to increased phagocytosis and cytokine production [28, 29]. Triterpenes exert anti-inflammatory, anticancer, and antioxidant effects through various biochemical pathways, including NF-κB inhibition, apoptosis induction, and free radical neutralisation [20].
In addition to terpene and carbohydrate compounds, the composition of Fomitopsis betulina includes sterols, fatty acids, alcohols, ketones, aliphatic aldehydes, vitamins, carotenoids, and phenolic acids [6, 10]. Some of these substances have verified biological activity of various kinds. This is why the studies also lead to demonstrating their synergistic effect in extracts from Fomitopsis betulina mycelium and fruit bodies. A cumulation of the compounds pre-sent in the extract results in a combination of molecules that may have complex anti-cancer effects as well as anti-inflammatory and anti-oxidative properties [6, 30]. In extracts obtained from Fomitopsis betulina, even compounds with a low biological activity can enter various interactions, resulting in a stronger therapeutic effect or causing a synergistic reaction of the body on the physiological level. For this reason, it appears to be much more beneficial to study this fungus species as a whole, without isolating individual substances or compounds, to better learn its therapeutic potential.
Understanding the bioavailability and LADME processes of compounds isolated from Fomitopsis betulina
Despite the widespread use of Fomitopsis betulina in traditional medicine, few studies have been conducted on the bioavailability and LADME (liberation, absorption, distribution, metabolism, excretion) processes related to this mushroom or its isolated compounds [31]. Bioavailability refers to the degree and rate at which an active substance or its active metabolite becomes available at the site of action after administration. It is a measure that indicates what portion of the administered dose of a drug reaches systemic circulation and is available to exert its therapeutic effect. Bioavailability is expressed as the percentage of the administered dose that reaches systemic circulation in an unchanged form. Key aspects of bioavailability include absorption, which is the process by which the active substance passes from the site of administration (e.g. the gastrointestinal tract) to systemic circulation, and the first-pass effect through the liver, where part of the drug may be metabolised, affecting bioavailability [32, 33].
Studies on mushrooms similar to Fomitopsis betulina suggest that metabolites may be biologically active, but specific studies on the metabolism of these compounds are limited [34]. Compounds isolated from Fomitopsis betu-lina, with potential anticancer activity, may include triterpenes (e.g. betulinic acid, betulonic acid, betulin), polysaccharides (β-glucans, heteroglucans), sterols (ergo-sterol), as well as phenols and flavonoids (p-hydroxybenzoic acid, protocatechuic acid) [6].
Terpenes often have low oral bioavailability due to their hydrophobic nature, which hinders their solubility in water and absorption in the gastrointestinal tract [35]. Betulinic acid, because of its hydrophobic properties, may struggle to dissolve in water, affecting its release from oral formulations [34]. To enhance absorption, various strategies are employed, such as lipid-based formulations, nanoemulsions, and nanoparticles [36]. Betulinic acid undergoes metabolism in the liver, resulting in various metabolites that may exhibit biological activity [37]. Studies on the metabolism of this acid indicate the formation of several metabolites, including betulonic acid. Cytochrome P450 enzymes are involved in betulinic acid metabolism [38].
Polysaccharides are large, complex molecules, which can affect their absorption and bioavailability in the body [39, 40]. Polysaccharides from mushrooms are typically extracted using water or aqueous solvents, which facilitates their release from oral formulations [39]. They are water- soluble, aiding their distribution in the gastrointestinal tract. Polysaccharides are usually poorly absorbed in the small intestine due to their large molecular weight and complex structure. However, some polysaccharide fragments can be partially hydrolysed by intestinal enzymes or gut microbiota, leading to the formation of smaller units that are better absorbed [40-42]. Polysaccharides can be fermented by gut microbiota into short-chain fatty acids (SCFAs) such as acetic, propionic, and butyric acids. Once absorbed, smaller polysaccharide fragments and their fermentation products can be transported to various tissues through the bloodstream [42, 43]. The immunomodulatory effects of polysaccharides may result from their interaction with immune cells in the gastrointestinal tract and beyond. The primary part of polysaccharide metabolism occurs in the colon, where they are fermented by gut bacteria. The resulting SCFAs can modulate energy metabolism and immune response. Polysaccharide metabolism products, such as SCFAs, can affect various metabolic pathways in the body, including lipid and glucose metabolism [43].
Further research is necessary to better understand these aspects and determine the potential therapeutic applications of compounds isolated from this mushroom [11].
Impact of extraction methods on the cytotoxic activity of Fomitopsis betulina
The method of preparing an extract affects its cytotoxic activity and is tied to the type of solvent used [10]. The in vitro studies conducted using an ethanol extract, an aqueous one, and a mixture of the 2, demonstrated an improved cytotoxic effect of the ethanol extract against the human malignant melanoma (A375) and melanoma (Hs895) cell lines, compared to the other 2 [23]. The effects of this ethanol extract were confirmed by later studies. It inhibited the viability of A375 cells, and to a lesser degree the primary melanoma (WM115) and human fibroblast (Hs27) cell lines [13].
Compared to the ethanol extract prepared from Fomitopsis betulina, the ether extract has a superior cytotoxic effect on large intestine adenocarcinoma [16]. A difference between those extracts was shown when prepared with the same solvent but using fragments taken from different parts (fruit bodies, mycelium culture) of the fungus. This study demonstrated a higher cytotoxic activity of the extract prepared from Fomitopsis betulina mycelium cultures compared to the fruit body extract from this fungus. The biomass extract had a better effect on the prostate cancer cell line (Du145) and showed selectivity towards the normal prostate epithelium cells cell line (PNT-2), on which the fruit body extract had a toxic effect. Both extracts showed no cytotoxic effect towards the normal skin fibroblast cells cell line (BJ). Against the melanoma cells (A375 and WM795), which differ in their metastasis potential, a better effect was observed for the mycelium extract, with the effect being weaker against the WM795 cell line, against which the fruit body extract had no effect at all [28].
Biological properties of Fomitopsis betulina confirmed by in vivo studies
In vivo studies confirm the biological properties of the fungus in question. Per os administration of hydrolysates from Fomitopsis betulina and Inonotus obliquus (Ach. Ex Pers Pilàt) to female dogs with a confirmed lactic gland tumour reversed the neoplasms and caused a liquefactive necrosis of the cancer cells. The clinical condition of the animals improved overall, resulting in increased appetite and body mass [44, 45]. To demonstrate the effect of the triterpenes present in Fomitopsis betulina on Sticker tumours in female dogs, the animals were administered per os an ether extract from the fungus. A receding was observed, and sometimes the disappearance of the tumours located in the vagina. As before, the condition of the animals improved overall while bleeding from the reproductive tract ceased. The extract did not affect tumours located near the nipples [24].
In 1995, Poznański et al. demonstrated a reduction in piglet mortality during nursing after administration of a preparation containing Fomitopsis betulina metabolites and a preparation containing a mixture of products of brown coal degradation by this fungus and metabolites of the microorganisms produced during this degradation. The latter also contributed to the improved growth rate for the piglets. The tested preparations did not affect the results of the haematology tests [46]. In their follow-up studies, the same authors demonstrated the immunosuppressive effect of Fomitopsis betulina metabolites. The preparation inhibit-ed the production of antiovoalbumin antibodies in piglets during nursing. For the preparation based on the products of brown coal degradation by Fomitopsis betulina and the metabolites produced in this reaction, the humoral response of ovoalbuminimmunised piglets was the same as in the control group [47]. Tests with Wistar male rats demonstrated that per os administration of organic extracts from Fomitopsis betulina caused no lesions in the circulatory system of these animals [31]. Despite positive reports on the biological and cytotoxic activity of Fomitopsis betulina extracts, we find few studies conducted with the use of animals.
The impact of environmental factors on fungal composition and future research directions
Anthropogenic factors, such as air and soil pollution, affect tree breathing processes and morphology. This contributes to lowering of the plant’s immunity, making it more susceptible to diseases and pests [48]. For this reason, it is important to study the composition and compound contents within individual species of fungi from different ecosystems. This is because the environment and nutrient access can affect the composition of the mycelium culture, and consequently the fruit body composition.
Another method of acquiring biologically active compounds from fungi is to use mycelium culturing under in vitro conditions. This enables the environment to be shaped in a manner that leads to the most effective synthesis of metabolites with pro-health properties and their further accumulation. This is made possible by adjusting the temperature and the pH of the substrate, and using the right sources of carbon and nitrogen etc. [49].
It is also possible to produce fungus fruit bodies under artificial and controlled conditions. Pleszczyńska et al. presented a successful production of mature fruit bodies of Fomitopsis betulina on a substrate made of birch sawdust, which was supplemented with organic additives [50].
Future research should focus on the impact of different environmental conditions on the composition of fungal metabolites, optimising in vitro and artificial culturing methods to enhance the production of beneficial compounds, and investigating the potential health benefits and applications of these compounds.
Limitations and potential solutions
So far, there are no clinical studies confirming the efficacy and safety of Fomitopsis betulina in humans. Moreover, in vitro studies are characterised by significant differences in extraction methodology and cytotoxicity assessment, which complicates the comparison of results from different studies. This is a relatively fresh topic, as indicated by the small number of included studies, all of which are from the last 5 years. Only 5 studies met the inclusion criteria, which may affect the completeness and representativeness of the review. The limited number of studies available and the variability in extraction methods further hinder the ability to draw definitive conclusions. Different extraction techniques can yield extracts with varying bioactive compound concentrations, potentially impacting the observed cytotoxic effects and making direct comparisons across studies challenging.
To increase the chances of developing the most favourable in vitro culture of this fungus for future in vivo studies and potential clinical trials involving humans, it is necessary to standardise the methods of preparing extracts from Fomitopsis betulina and the standards for conducting studies. The antitumour activity of compounds of natural origin has been reported thousands of times and proven by many natural extracts; however, the medical usefulness in clinical cancer treatment is generally weak. The action is often cytostatic rather than cytotoxic, and generally terpenes and glucans are not strong enough to replace conventional anticancer chemotherapy.
Furthermore, the action of individual compounds may be weaker than the synergistic action of all compounds contained in Fomitopsis betulina. A potential solution is to consider these compounds with the utmost caution as chemopreventive agents, supporting conventional therapy. Integrating Fomitopsis betulina into existing treatment protocols could improve the effectiveness of anticancer therapies while minimising the side effects of conventional chemotherapy.
Conclusions
The reviewed studies highlight the promising cytotoxic potential of Fomitopsis betulina extracts and isolated compounds, particularly against cancer cell lines. Across the 5 analysed papers, varying extraction methods (methanol, ethanol, and water) demonstrated selective cytotoxic effects on cancer cells while sparing normal cells. Triterpenes and glucans were consistently identified as the bioactive compounds responsible for these effects, with specific focus on prostate cancer, melanoma, and colorectal cancer cell lines.
However, significant variability in extraction methods and cytotoxicity assays between studies complicates direct comparison of the results. While some extracts exhibited notable selectivity, others showed limited differentiation between normal and cancerous cells. The limited number of studies, all published within the last 5 years, further restricts the ability to draw comprehensive conclusions. Standardisation of extraction methods and cytotoxicity testing is crucial for future research to provide more robust and comparable data.
In summary, Fomitopsis betulina exhibits potential as a source of selective anticancer agents, but further studies – especially in vivo research and clinical trials – are essential to validate its therapeutic efficacy and understand its mechanisms of action.
Studies included in quantitative synthesis: 5