Introduction
Growth hormone has been licensed for usage in children with growth hormone deficiency (GHD) since 1983. Since then, growth hormone therapy has come a long way, including FDA-approved usage for numerous adult and paediatric conditions. Often, cost and feasibility are barriers in a developing country like India. Data on determinants and response to growth hormone therapy is plentiful from the west because the cost of therapy is borne by public health care systems in these countries [1–3]. Children diagnosed as having short stature have to be evaluated meticulously to arrive at a proper diagnosis of hypopituitarism [4–8]. Data on responses in Indian children are available from Northern India and Western India [9–13]. There is a paucity of data in this regard from the southern part of the country. Knowledge on the spectrum and response to growth hormone will help paediatricians in our setting to counsel and convince the patients to undergo growth hormone therapy.
With this background, we performed this study with the aim of assessing the response of South Indian children with growth hormone deficiency to growth hormone therapy, and factors determining the response of our children to growth hormone therapy
Material and methods
We consecutively recruited non-acquired GH-deficient children referred to a tertiary-level paediatric endocrine care unit in Chennai, India over a period of one year. The diagnosis of GHD was based on short stature (<−2.0 SD below the mean height for age- and sex-matched children) and failure to show serum GH concentrations above 10 µg/l after one provocation test using clonidine (0.15 mg/m2) or glucagon (0.03 mg/kg) as stimulating agents prior to enrolment in the study [4–8]. All pre-pubertal boys > 11 years and pre pubertal girls > 10 years were primed with intramuscular testosterone 50 mg 3 days prior to test and oral oestradiol valerate 2 mg orally for 2 days, respectively [8]. We recruited children with isolated GHD and combined pituitary hormone deficiency (CPHD, 2 or > 2 hormone deficiencies). Subjects with CPHD were on stable substitution therapy with thyroxine (100 µg/m2/day) and/or hydrocortisone (10 mg/m2/day) and or D-Desamino Arginine Vasopressin spray 10 µg/day (titrated as per urine output). Children with dysmorphic syndromes, chromosomal abnormalities, and acquired causes for GHD like tumours and trauma were excluded from the study [4–8].
Detailed history pertaining to birth weight, mode of delivery, neonatal events, and family history was elicited and entered in the predesigned data entry card. Height, weight, and body mass index (BMI) were assessed as per standard protocols. The anthropometric measures were converted into Z-scores based on WHO standards or IAP 2015 references, as appropriate [14, 15]. Pubertal status was assessed by a single paediatric endocrinologist. A detailed meticulous physical examination was performed to look for central defects, genitalia, micropenis, and other phenotypic features of GH deficiency.
Bone age was estimated in children over 5 years old by a single paediatric radiologist using the RUS score of Tanner Whitehouse 3 method (TW3 method) [16]. Serum GH concentrations were assessed by a solid-phase, two-site chemiluminescent immunometric assay with an intra assay coefficient of variation (CV) of 5.3% and inter assay CV of 5.5%. Magnetic Resonance Imaging (MRI) scans were performed in a 1.5 Tesla unit (Signa, GE, Milwaukee, WI) using T1-weighted sagittal and coronal scans with TR: 350 ms and TE:20 ms.
The recruited subjects were initiated on growth hormone on the basis of logistic considerations including affordability, willingness to undergo growth hormone therapy, and eligibility for government sponsored schemes for free growth hormone therapy. Children receiving growth hormone therapy were considered as cases (group I), and children not receiving GH considered as controls (group II).
Growth hormone therapy
Children in group I were treated with recombinant human growth hormone (rhGH) [Novo Nordisk Med India Pharma, Chennai, India or Head on from Sun pharma and Company Mumbai (India) Private Limited, India] for a period of 12 months (dose 0.07 IU/kg/day subcutaneously at night) with an injection frequency of 7 injections per week. The dose was escalated to 0.1 IU/kg/day based on auxological response and IGF-1 levels. The criteria for discontinuation of GH treatment were a growth velocity less than 2 cm/year in the previous 6 months with a bone age greater than 14 years for girls and 16 years for boys [4–8, 17].
All children were followed up every 3 months for compliance, site rotation and local reactions, adverse reactions, and auxology. They underwent biochemical assessment of fasting blood sugar, glycosylated haemoglobin, and concomitant pituitary hormone deficiency. Compliance to the medication was assessed by empty growth hormone cartridge count and pill count, assessed by empty bottles (for thyroxine and hydrocortisone) in every clinic visit. The compliance was also ensured by telephonic contact undertaken by the endocrine educators of the unit as a part of the study protocol. The families were instructed to maintain contact with the endocrine team every month during their therapy period.
The study was approved by the Institute Ethics Committee. Informed consent was obtained from parents before the study was commenced.
Statistical analysis
Data was entered into Excel 2007. The standard deviations (SD scores) of the anthropometric parameters as per the WHO standards (Anthro software) [14] and IAP 2015 references [15]. Growth velocity was converted into Z-scores using available published references [18]. Simple t-test and chi-square test were used to compare study parameters, as appropriate. Multiple linear regression analysis was performed to determine the factors likely to determine auxological improvement. Because target height SD scores can impact current height SD scores, we used Atkins scatter plots to compare the improvement in height SD scores in the 2 groups. Kaplan-Meyer survival analysis was performed to determine the duration of growth hormone therapy required to attain height SD scores in the target range. Data were analysed using IBM SPSS version 21.
Results
Out of 170 children with short stature seen during the study period, 55 children were diagnosed as GHD. We excluded 9 children – on the basis of presence of intracranial tumour in 3 and genetic diagnosis of Prader-Willi syndrome in 6 children. Finally, 46 children were recruited to the study. Of these 46 children, 23 children opted to take growth hormone for a period of 1.8 ±0.3 years, for a minimum period of 1 year (recruited as cases, group I). The remaining 23 children did not take growth hormone owing to logistical problems (recruited as controls, group II) and were followed up in the endocrine clinic. Baseline demographic data pertaining to clinical, auxological, biochemical, and radiological parameters in the 2 study groups are presented in Table I.
Table I
Comparison of baseline demographic, clinical, auxological, biochemical, and radiological features in groups with and without GH treatment among children with GHD
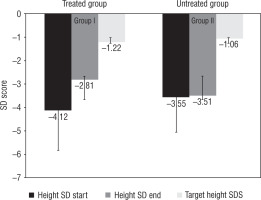
The subjects in group I (treated with GH) had a significant improvement in height SD score from –4.12 ±1.7 to –2.81 ±1.52 (p < 0.05) compared to group II (untreated group) –3.55 ±1.7 to –3.51 ±1.52 (p > 0.05). The corresponding values for weight and BMI SD scores were –3.55 ±2.21 and –1.52 ±1.65 in group I and –2.7 ±1.2 and –1.77 ±1.2 in group II. Parallel to an increase in the height, SD score, and growth velocity, we observed one child (4.7%) in group II who entered puberty and 8.7% in group I who entered into puberty baseline – progressed in puberty (p > 0.05) (Fig. 1).
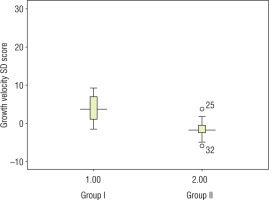
Growth velocity among treated children in group I (13.2 ±5.6 cm/year, SD score 2.5 ±1.4) was significantly higher as compared to untreated children in group II (3.4 ±1.8 cm/year, SD score –1.62 ±2.38) (Fig. 2). Other clinical benefits we observed with growth hormone during follow-up visits included the following: better school performance, peer interaction, and reduced frequency of infections, in one child each.
To determine the factors likely to impact the response to growth hormone therapy in group I children, a univariate analysis was performed. Age of onset of GH therapy and duration of GH therapy were found to be significant determining factors. To determine independent factors that are likely to impact response assessed by growth velocity, a multivariate regression analysis was performed. It was observed that duration of growth hormone therapy, presence of ectopic posterior pituitary, and BA : CA ratio independently impacted the growth velocity SD scores (p < 0.05). Presence of hypoplastic pituitary, target height SD score, sex, baseline height SD score, and chronological age did not impact growth velocity SD scores (p > 0.05).
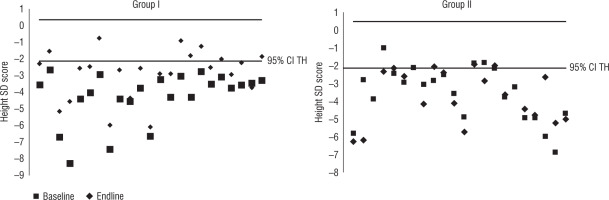
To further improve our understanding of the catch up in the two groups, an Atkins scatter plot of height SD score and the disparity of the SD score between the measure and the target height SD score was constructed (Fig. 3). It was observed that more readings in group II fell out of the 95% confidence limits compared to those of group I (p < 0.05).
Figure 3
Comparison of increment in height SD scores versus target height SD scores in groups I and II (Bland Altman plot)
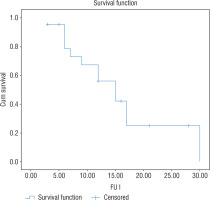
A Kaplan-Meier survival curve was constructed to estimate the cumulative probability of development of height SD scores within the target height range (±2 SD) across the duration of GH therapy. It was seen that 15 months was the mean duration of GH therapy needed to attain height in the ±2 SD of target height (Fig. 4)
Figure 4
Kaplan Meier survival analysis curve demonstrating height SD score within 2 SD score of target height range
None of the children in group I developed any adverse reactions to growth hormone therapy. The injection sites remained healthy during the study period. Monitoring of blood sugar revealed no abnormality during monitoring at follow-up visits. None of the children discontinued therapy during the period of study. All families stored the medication appropriately in a refrigerator.
There was no mortality during the period of study.
Discussion
Ours is a South Indian study showing the response to GH therapy among children with GHD. Our study suggests strongly that GH therapy significantly improves the auxology (both height SDS and growth velocity SD score) among South Indian children with growth hormone deficiency.
The increment in height SD score observed by us (–4.1 to –2.8) is similar to that reported by Bajpai et al. [9] from New Delhi (–5.1 to –3.4) and Khadilkar et al. (–4.8 to –3.4) [10]. Improvement in growth velocity (13.25 ±5.6 cm/year) was significantly higher than other studies Khadilkar et al. (12.1 ±2.8 cm/year), 8.7 ±2.7 cm in the first year by Garg et al. [9], and 9.2 ±2.3 cm/year KIGS cohort [19]. The possible explanation for better growth velocity includes better compliance and adherence to the regimen (assessed by meticulous follow-up in our study), younger age of initiation of therapy in our study, early age of diagnosis (baseline height SD score in our study is –4.1 versus –4.8 from Khadilkar et al. and –5.1 from Bajpai et al.). Also, we do not know the genetic mutation of our children with GHD because genetic mutation may influence the response to GH therapy [20]. A recent long-term study on 99 children from North India revealed that growth velocity to growth declined from 10.6 ±3.0 cm/year in the first year of therapy to 8.7 ±2.7 cm/year (in 2nd year) and 7.9 ±2.2 cm/year (in 3rd year), and to 4.8 ±3.6 cm/year during the 7th year [13].
The 3 important predictors of improved response to GH (demonstrated by growth velocity SDS) were duration of therapy, presence of ectopic pituitary, and delayed bone age (assessed by BA:CA ratio). Duration of therapy and delayed skeletal maturation are in agreement with previous observations [4–6]. Longer duration of growth hormone therapy results in higher production of IGF-1 from the liver, resulting in higher anabolic action, in turn resulting in better growth velocity. Degree of delayed bone age – a surrogate marker for severity and duration of GH deficiency and a reflection of the remaining growth potential – is directly related to the augmentation of height. It is worthwhile to note that our series of children were predominantly younger and more often pre-pubertal than most other Indian series [9–13]. Thus, these two factors are pivotal determinants of growth response. The impact of pubertal hormones on growth would be minimal at this stage.
Table II
Regression analysis of factors affecting growth velocity in the growth hormone-treated group
Zenaty et al. [21] observed that the degree of response to GH is related to abnormalities in the MRI. Ectopic posterior pituitary tissue could result from defective neuronal migration during embryogenesis or a transection of the pituitary stalk followed by hypertrophy of the proximal axons with subsequent reorganisation. A better response in children with ectopic posterior pituitary in our study may be of added value while paediatricians counsel parents for GH therapy.
Growth in children is multifactorial, including genetic, nutritional, and endocrine factors. Interpretation of anthropometry as Z-scores annuls the effect of age and sex, but target height (genetic potential) can significantly impact the current height of a child. The target heights in groups I and II are comparable. To further prove that the improvement in auxology is indeed related to GH therapy an Atkins plot was done, which revealed that more readings fell out of the 95% CI for the target height range in group II versus group I (at the end of the follow-up period).
A Kaplan-Meier survival curve (considered as a gold standard to study the dynamics of catch up) constructed to estimate the cumulative prevalence of development of height SD scores in target height range (±2 SD) was 15 months of growth hormone therapy. This duration is substantially lower than the 20 months observed by Bajpai et al. [9]. The mean age of GH initiation was 9.9 ±3.7 years in the Bajpai et al. study versus 4.5 ±3.3 years in our study. This emphasises the need for early diagnosis and early initiation of GH therapy, so height close to target height can be attained with a shorter duration of GH.
It is well known that growth in children with IGHD and appropriately treated CPHD is comparable in terms of initial response to GH and final height achieved [22]. Furthermore, there were no differences in study parameters in children with IGHD and CPHD at the onset of study. Hence, children with IGHD and CPHD (on stable substitution therapy) were considered as one group for analysis. We combined subjects treated with biosimilar growth hormone on par with conventional growth hormone [23]. It is recommended that 2 GH stimulation tests to be done prior to diagnosing GHD because it gives better specificity. We adopted 1 as clinically appropriate for logistic reasons. This has been the practice in developing countries including many centres in our nation; also, the auxology, delay in bone age, low IGF-1, and MRI pituitary give substantial confirmation of diagnosis of GHD [24]. In the current study, we do not have a long-term final height attained by children. Hence, the catch up observed in our study – whether it is sustained till adult height is reached – needs to be followed up for a longer period.
Thus, we conclude that growth hormone therapy is indeed feasible in our setting and provides optimal response. Early diagnosis, pre-pubertal status, delayed bone age, and presence of ectopic posterior pituitary on MRI are determinants of a better response. Growth hormone must be administered for at least 15 months to reach height within the target range in the short term, if diagnosed early.

 ENGLISH
ENGLISH