A 67-year-old male smoker with hypercholesterolemia and hypertension was admitted to the cardiology department due to ST-segment elevation myocardial infarction (STEMI). An electrocardiogram (ECG) revealed T-wave inversion in V1-V6 and ST-segment elevation in leads V2-V5, indicating anterior wall STEMI. Coronary angiography (EBU 3.5 6F catheter) revealed dissection of the aortic root and proximal part of the ascending aorta (Figure 1 A). The patient remained stable and asymptomatic, with blood pressure 120/65 mm Hg and a heart rate of 70/min. The Heart Team (HT) decided to defer primary revascularization due to the risk of dissection progression and perform computed tomography (CT) with coronary angiogram to assess the extent of dissection and the severity of coronary artery disease. Transthoracic echocardiography ruled out pericardial effusion. Follow-up ECG showed ST-segment resolution. The CT revealed contrast medium extravasation in the aortic root wall, confirming intramural hematoma (IMH) diagnosis (Figure 1 B). CT coronary angiogram showed 70–80% narrowing of the medial left anterior descending (LAD) artery (Figure 1 C). Based on the clinical presentation and risk of IMH progression, revascularization was deferred. CT performed the following day revealed stabilization of the aorta wall. HT decided to continue the conservative treatment. Blood investigations showed increased troponin levels in consecutive assessments: 8745.9 pg/ml (baseline), 12617.6 pg/ml, 15202.9 pg/ml and 3766.7 pg/ml (norm 0–34.2 pg/ml). The asymptomatic patient was discharged home on dual antiplatelet therapy (aspirin and clopidogrel), with coronary angiography scheduled in 5 months. Five months later, the HT opted for cardiac magnetic resonance (CMR) over coronary angiography, due to prior complications during coronary angiography (IMH) and the patient’s asymptomatic status. The stress CMR showed postischemic damage of the left ventricle with a significant, reversible perfusion deficit and thickening of the anterior wall of the ascending aorta, corresponding to the previous IMH. Based on these findings, HT scheduled percutaneous coronary intervention (PCI) in 3 months. It was performed using a Judkins Left 4.0/6F catheter and one 2.75 mm/18 mm drug-eluting stent was implanted in the proximal segment of the LAD (Figures 1 D, E). The asymptomatic patient was discharged on current drug treatment therapy.
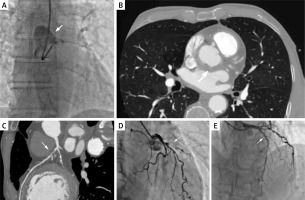
Figure 1
A – Coronary angiography: dissection of the aortic root and proximal part of the ascending aorta. B – Axial computed tomography: intramural hematoma in the ascending aorta. C – Coronary computed tomography: 70–80% occlusion of proximal left anterior descending artery. D – Coronary angiogram: subtotal stenosis in proximal left anterior descending artery. E – Coronary angiogram: the same segment after stent implantation
STEMI with concurrent IMH is relatively rare, but associated with frequent complications [1]. The overlapping symptomatology may lead to misdiagnosis and inappropriate management. CT identifies two IMH types: ulcer-like projection and intramural blood pool. The latter has a better prognosis and often regresses without complications. IMH thickness over 10 mm and its increase in the following CT are associated with poor outcome [2]. In our case neither of these features were present, supporting deferred PCI. Recent studies indicate the possibility of stenting as iatrogenic aortic dissection’s treatment [3]. In our case the conservative approach was chosen due to the asymptomatic course and favourable type of IMH. Additionally, the IMH was revealed during contrast medium administration prior to coronary artery catheterization, suggesting non-iatrogenic etiology. The 8-month follow-up demonstrated the safety of conservative treatment without symptom deterioration.
Coexistence of STEMI and IMH in the ascending aorta poses a diagnostic and management challenge. In selected asymptomatic patients without IMH progression, deferment of interventions can be a safe approach.








