Introduction
The Bretschneider intracellular cardioplegia has been widely used since the 1970s as a single-infusion cardioplegic solution in various cardiac procedures [1]. Its efficacy has led to extending the usage for organ preservation in transplant procedures. The evidence is quite convincing, as some studies report myocardial protection for a period of up to 3 h [2, 3]. As such, it is a primary choice in many experienced cardiac centres providing surgical treatment for complex procedures.
However, alternative solutions for one-shot cardioplegia administration may be used. The interest in the del Nido solution in adult cardiac procedures has been constantly growing. Its cost efficiency and satisfactory clinical profile have already been proven in randomized clinical trials comparing the solution with blood cardioplegia [4, 5]. Although only a non-significant trend towards reduced troponin release was visible, some indirect indicators of myocardial protection (such as occurrence of post-clamp ventricular fibrillation) seemed superior in the del Nido group. The del Nido cardioplegia is claimed to ensure satisfactory protection for as long as 90 min of cross-clamp [6].
To date, no randomized comparison of the del Nido and Bretschneider solution has been published. To address this matter and provide a rationale for a larger, randomized trial, a case-control matched study in an aortic valve replacement group was performed. The isolated aortic valve replacement patients were selected as they represent a group of very similar clinical profile. The procedures are easily reproducible and extracorporeal circulation is conducted in a similar way in most of those cases. This allows one to assess the most detailed aspects of providing one solution or another, including biomarker release, rhythm disturbances or electrolyte imbalance.
Aim
To determine the efficacy of the del Nido cardioplegia when compared to Bretschneider HTK solution in patients undergoing aortic valve replacement for severe aortic stenosis.
Material and methods
Patients
Ten patients undergoing isolated aortic valve replacement for severe aortic stenosis using the Bretschneider HTK solution were propensity matched to patients undergoing AVR using the del Nido solution. Matching parameters included age, gender and cross-clamp time.
Cardioplegia
The Bretschneider Histidine-Tryptophan-Ketoglutarate cardioplegia was provided as a commercially prepared solution. The component concentration was as follows: sodium – 15 mmol/l; potassium – 9 mmol/l; magnesium – 4 mmol/l; calcium – 0.015 mmol/l, ketoglutarate/glutamic acid – 1 mmol/l; histidine – 198 mmol/l; mannitol – 30 mmol/l; tryptophan – 2 mmol/l. The cardioplegia was administered in a dose of 1–2 l at a target flow of 200–300 ml/min and a temperature of 4°C. Due to a high volume given in a single administration, furosemide in a dose of 40 mg was given additionally.
The del Nido cardioplegia was prepared with a calculated doses to provide a solution identical to that originally described (Plasma-Lyte A – 1000 ml; mannitol 20% – 16.3 ml; MgSO4 50% – 4 ml, NaHCO3 8.4% – 13 ml, KCl 2 mEq/ml – 13 ml, lidocaine 1% – 13 ml). This solution was mixed with patient’s blood from the cardiopulmonary circuit in a 4 : 1 ratio (crystalloid/blood). The component concentrations were as follows: sodium – 142–144 mEq/l; potassium – 24 mEq/l; calcium – 0.22–0.27 mmol/l; magnesium – 15 mEq/l. The dosage was 20 ml/kg, the target administration pressure was 100 to 200 mm Hg and the target administration flow was 200 to 300 ml/min. The solution temperature was 4°C. Due to the high volume in a single delivery, 40 mg of furosemide was injected into the cardiopulmonary circuit.
Procedure
The aortic valve replacement was performed through median sternotomy. After accessing the pericardium, the heparin was given. The aorta was cannulated, as well as the right atrium (double-stage venous cannula). The vent was inserted into the left ventricle through the right superior pulmonary vein. The cardioplegia was given antegradely. Following the cardioplegia administration, the aortotomy was made and native aortic valve was removed. The prosthesis (mechanical or bioprosthesis) was implanted using single sutures with pledgets. The aortotomy was closed and deairing manoeuvres were performed. The cross-clamp was removed. If required, a defibrillation was performed for a fibrillating the heart. Two epicardial electrodes and one or two chest tubes were inserted. The chest was closed.
Parameters
The intraoperative observation was performed to record cardioplegia dosage, time from the beginning of cardioplegia administration to cardiac arrest, cross-clamp time, extracorporeal circulation time, presence of cardiac rhythm during the cross-clamp, ventricular fibrillation after the cross-clamp and gasometry parameters after removal of the cross-clamp.
The postoperative observation was performed to record creatine kinase (MB isoenzyme – CK-MB) at 24 and 48 h following the surgery and troponin (highsensitivity troponin T – hsTnT) at 24 and 48 h following the surgery. The reference values for biomarkers are provided in the tables. Furthermore, observation of postoperative ejection fraction, heart rhythm disturbances (particularly atrial fibrillation) and peak creatinine values was conducted.
Statistical analysis
The analysis was performed with MedCalc v.18.5 (Med-Calc Software, Ostend, Belgium). Data are presented as median (interquartile range) or number (percentage). As the Shapiro-Wilk test rejected the normal distribution, the Mann-Whitney U test was used for continuous parameters. The Fisher exact test was used for categorical data analysis. A p-value of ≤ 0.05 was considered statistically significant.
Results
The propensity matched patients (criteria: age, gender and cross-clamp time) were no different in terms of comorbidities (diabetes, hyperlipidaemia, smoking, hypothyroidism, asthma/COPD, cerebrovascular incidents) ventricular function and native valvular parameters (Table I).
Table I
Preoperative parameters. Data are presented as number (percentage) or median (interquartile range)
All of them underwent the aortic valve replacement procedure with a single infusion of cardioplegic solution. Most of the intraoperative parameters were not different. However, there was a significantly higher number of ventricular fibrillation incidents in the Bretschneider solution group (Table II).
Table II
Intraoperative parameters. Data are presented as number (percentage) or median (interquartile range)
In contrast to the del Nido solution, most of patients in the Bretschneider group presented with hyponatraemia after removing the cross-clamp. Furthermore, lower glycemia values were visible in the del Nido group, but the difference was not significant (Table III).
Table III
Gasometry parameters after removing the cross-clamp. Data are presented as median (interquartile range)
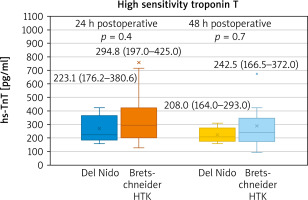
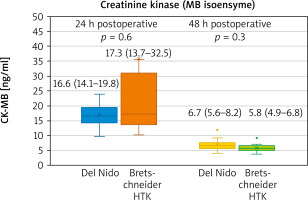
In the postoperative period there was no statistical difference in analysed parameters. However, slightly lower peak creatinine values were present in the Bretschneider group (Table IV). The biomarker release was similar in both groups, with a non-significant difference slightly favouring the del Nido cardioplegia (Figures 1, 2).
Table IV
Postoperative parameters. Data are presented as number (percentage) or median (interquartile range)
Discussion
Although single-administration cardioplegic solutions are particularly required in complex or minimally invasive surgery, some observations can be made successfully in more simple procedures. Aortic valve replacement for severe aortic stenosis serves that purpose ideally. First of all, patients represent a group that is equal in terms of clinical profile. Furthermore, although the procedure is characterized by rather short cross-clamp time, the cardioprotection may be demanding due to hypertrophied myocardium and alterations in endomyocardial perfusion.
Taking the intraoperative parameters into analysis, a significantly higher number in ventricular fibrillation following cross-clamp removal becomes apparent. VF is a result of biochemical processes that lead to energy depletion, which proceeds despite the reduction of subendocardial perfusion. Advancing acidosis worsens the damage [7, 8]. The defibrillations aggravate the injury [9]. Of note, in a recent systematic review the authors found that there was a trend for increased incidence of VF that reached statistical significance in the fixed but not the random effects model and that six of the eight studies reported a higher incidence of VF after removal of the cross clamp in the Bretschneider group [10]. As such, the endpoint regarding ventricular fibrillation following the cross-clamp remains the important aspect of analysis. In a randomized trial comparing the del Nido and multidose cold blood cardioplegia protocol a doubt regarding lower incidence of VF was visible – it might have been associated with progressive rewarming in the del Nido group with multidose cold blood cardioplegia, but also with addition of lidocaine [5]. However, the first of those reasons does not concern the current analysis, as both solutions are administered only once. Seeing lower biomarker release in the del Nido group, although not reaching significance, larger randomized analysis appears even more justified.
The aspect of electrolyte imbalance after removing the cross-clamp (gasometric analysis) is often underestimated, but may also be prognostic in terms of clinical outcome. In their report, Crestanello et al. revealed that postoperative hyponatraemia was associated with an increased risk of morbidity and mortality [11]. This association was valid after adjusting for demographic, preoperative and operative factors. As such, electrolyte imbalance following cross-clamp should be of the surgeon’s interest. Significantly lower sodium values in the Bretschneider cardioplegia group require confirmation in a larger trial. Importantly, both cardioprotection protocols present with relatively low and similar values of calcium ion. This is an important observation, as calcium ions are currently believed to be key factor in ischemia-reperfusion injury [12].
Interestingly, glucose values appeared to be lower in the del Nido group, despite the fact that there were four diabetics in the del Nido group compared with one in the Bretschneider HTK group. Although statistical significance was not reached, it might become apparent in a larger trial. Some reports already indicate that the del Nido solution can be used safely and effectively as an alternative to Buckberg solution in adult isolated valve surgery and is associated with lower insulin requirements [13]. Randomized comparisons of the del Nido and blood cardioplegia solutions also reveal a trend towards lower postoperative glycemia in the del Nido cardioplegia [5].
Although it is difficult to detect true differences in cardiac injury of cardioplegic arrested hearts, biomarker release remains one of the key indirect markers of myocardial damage [14]. The slight trend favouring the del Nido cardioplegia along with lower incidence of ventricular fibrillation in the del Nido group requires further investigation. Of note, when compared to a multidose blood cardioplegia, the del Nido solution presented with a visible trend but no statistical significance [4, 5]. In studies comparing the Bretschneider HTK with a conventional cardioplegia model, mean differences for both CK-MB and TnI did not differ between groups [11].
Similar incidence of postoperative arrhythmias is an important observation that should be included in a potential trial, as the arrhythmia contributes to the worsening of the postoperative state and prognosis, increases the length of intensive care unit stay and raises hospital costs considerably [15–17]. In studies comparing Bretschneider with conventional cardioplegia, there was no significant difference in the rate of AF between groups [11]. The same applies to the del Nido cardioplegia reports [5].
The significance of the difference in postoperative maximal creatinine was not shown, but a non-significant difference favouring the Bretschneider cardioplegia is visible. This matter should be investigated further, as surgery-related kidney injury is the second most common cause of acute kidney injury in the intensive care setting (after sepsis) and is independently associated with increased morbidity and mortality [18].
Conclusions
Both del Nido and Bretschneider provide adequate myocardial protection, which has been already proven in clinical trials. However, one solution may be superior to another in a particular clinical situation. As such, analysis of clinical outcomes and detailed analysis of cardioprotection aspects are required. Differences in the presented pilot study provide the rationale for a larger, randomized clinical trial with some of the analysed parameters as reasonable endpoints.