Introduction
The first clinical manifestation of allergic purpura was described by English physician William Heberden in 1806, which included purpuric rash, arthralgia and abdominal pain [1]. The association of non-thrombocytopenic purpura and joint pain was described by German physician Johann Schönlein in 1837 [2]. Eduard Henoch added gastrointestinal and renal involvement in 1874 [3]. Therefore, allergic purpura is also called Henoch-Schönlein purpura (HSP). In recent years, HSP has been defined as a systemic vasculitis characterized by the deposition of immunoglobulin A (IgA)-containing immune complexes on the walls of the venules, capillaries, and arterioles [4, 5]. HSP generally affects children between the ages of 5 and 15 years and is more frequent in boys than in girls. The mobility in spring was much higher than that in other seasons [6, 7].
According to the clinical manifestations, HSP is divided to 5 types [8-11]: 1) skin type: it is also called simple purpura. The clinical manifestations are listed as follows: purpura distributed symmetrically over the extensor surfaces of the lower limbs, post aurem and perineum; patients do not have the symptoms of painful or itchy spots; rash disappears several days later, but it could also reappear in batches; rash could confluent to be a blood blister; necrosis could occur in the center; purulent secretion could be observed in a complicated rash infection; 2) joint type: besides the rash referred to above, some patients have the symptoms of joint swelling and pain. The joints include the knee, ankle, sacroiliac joints, even the elbow, wrist and digital joints. Tenderness and swelling of the skin could be detected; 3) gastrointestinal tract type: this type of HSP is the most common purpura in clinic. It is easily misdiagnosed as surgical abdomen or acute gastroenteritis. Patients reported diffused abnormal pain which is mainly located around umbilicus or hypogastrium. The pain is paroxysmal colic pain or persistent dull pain, often accompanied by nausea, vomiting, diarrhea or constipation. The character of this type of HSP is separation of clinical symptoms and signs, which means that the pain is severe while the sign is slight; 4) kidney type: it is also called Henoch-Schönlein purpura nephritis (HSPN), which often occurs 1 to 8 weeks after subcutaneous hemorrhaging. It may happen after the diagnosis of purpura. In some individuals, HSPN occurs before the diagnosis of purpura. The clinical manifestations are bloody urine, albuminuria, even cylindruria; 5) mixed type: this type of HSP consists of more than 2 items listed above.
Adult mixed-type HSP is not common in clinic. Few reported studies have focused on this type of HSP disease, which has been found worse clinical features and poor outcome in clinic generally. Early clinical manifestations of adult mixed-type HSP include abdominal pain, gastrointestinal tract bleeding and cutaneous purpura. Just taking the manifestations mentioned above as criteria, it is difficult to diagnose correctly in the early stage due to the lack of specificity [12-14]. Prior reported studies about diagnosis and treatment of adult HSP are limited by the number of event patterns, selection bias, and different definitions of disease severity and complications. Therefore, we conducted a retrospective study to explore better diagnosis and treatment for patients admitted with adult mixed-type HSP disease. We investigated the clinical manifestations and outcomes of patients with adult mixed-type HSP and imaging characteristics of the disease, and evaluated the efficacy of combined therapy in treating symptoms of HSP.
Material and methods
Patients
From January 2008 to October 2012, 23 adult patients (range: 16-54 years; 13 males and 10 females) with mixed-type HSP were enrolled in this study. The median age of all patients was 41.2 years. The diagnosis of HSP was made according to the American College of Rheumatology (ACR) 1990 criteria [15]. Criteria for inclusion in the study were a clinical diagnosis of HSP (i.e., typically distributed purpura with or without abdominal pain, joint pain and/or hematuria, proteinuria). Patients with vasculitis and purpura caused by other factors were excluded. The study was approved by the Ethics Committee in our hospital and the signed consent forms from all patients were obtained.
The basic clinical features such as age, sex, possible induced factors, and symptoms of the gastrointestinal tract, abdominal signs and simultaneous symptoms were recorded. The conventional examinations on blood white blood cells, occult stool blood and proteinuria were conducted. Positive proteinuria was considered if the urine protein was more than 350 mg/24 h detected by Beckman Coulter DXC800 (USA).
Abdominal contrast-enhanced computed tomography examination
Computed tomography (CT) examination was performed using a GE 64-layer spiral CT scanner (GE Light speed 64 VCT, United States). All patients underwent abdominal CT planar scanning first. The following CT scan parameters were set: tube tension 120 kV, tube current 36 mAs, collimation 40 mm, rack rotation time 0.6 s/circle, pitch 1.375 : 1, and slice thickness 3.0 mm. Then enhancement was achieved with 1.5 ml/kg body weight intravenous non-ionotropic contrast agent (Ultravist 370) injected at a rate of 3.5-4.5 ml/s to a peripheral vein.
Method for small intestinal enteroscopy
The enteroscope was inserted by an endoscope through the mouth to the inferior segment of the ileum. For patients with no bleeding lesion detected at one side, 0.5 ml of diluent methylene blue was injected beneath the mucous membrane at several sites. The methylene blue can facilitate confirming the site when the examination is performed through the other side [16, 17]. For some patients, X-rays were used to observe the extent of penetration by the enteroscope [18].
Renal needle biopsy
For all the patients with positive urine protein, ultrasonic guided renal needle biopsy with 18G biopsy needle was performed, immunofluorescence and pathologic examinations were performed according to the methods reported previously [19].
Therapy method
For patients with abdominal pain, normal saline prednisone (100 ml/d) plus dexamethasone (10 mg/d) once a day was used to relieve the symptoms of the gastrointestinal tract. The dosages were reduced following the relief of symptoms. About 5 days later, the dosage of dexamethasone was reduced to 7.5 mg for 3-5 days. Afterwards, it was reduced to 5 mg once a day for 3-5 days. Then patients were advised to take prednisone (40 mg/d) orally once a day. The course of the treatment was continued for 1-3 months. Patients with severe abdominal pain can be treated with 10 mg of anisodamine (654-2) which was added into 500 ml of saline prednisone for intravenous drip to relieve pain.
Results
Basic clinical information
The clinical manifestations of adult mixed-type HSP are listed in Table 1. The chief complaints of abdominal pain were obtained from 21 patients, including upper abdominal pain (3, 13.04%), umbilicus surrounding pain (9, 39.13%), right lower quadrant pain (2, 8.70%), hypogastralgia (7, 30.43%). However, only two cases did not point out the pain site. Except symptoms of the gastrointestinal tract described above, simultaneous phenomena like joint pain (14, 60.87%), leg pain (2, 8.70%) were also pointed out. Among these patients, 5 (21.74%) reported upper respiratory infection, 2 (8.70%) had taken drugs, 8 (34.78%) had eaten fish and shrimps, 8 (8.70%) showed intestinal infection, while the other 6 cases did not report any possible situations causing HSP. Abdominal signs observed for the total patients can be classified as follows: soft abdomen (13, 56.52%), slight tense abdomen (10, 43.48%), abdomen tenderness (18, 78.26%), rebound tenderness (2, 8.70%). A total of 19 patients (82.61%) showed white blood cell (WBC) of more than 10 × 109/l in laboratory examinations. In addition, 18 cases (78.26%) with positive stool occult blood and 21 cases (91.3%) with positive urine protein were exhibited. Blood clotting function of all the patients was normal.
Table 1
Clinical manifestations of adult mixed-type Henoch-Schönlein purpura patients
Typical skin manifestation
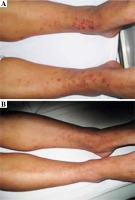
Purpuric lesions with different sizes were found in these patients. The distribution mainly involved dependent areas such as the lower extremities (Fig. 1A). Initially the lesions were single and little, but later coalesced to form ecchymotic areas. After treatment, the purpura disappeared and the color darkened. These results indicated a significant recovery for skin lesions after treatment (Fig. 1B).
Abdominal contrast-enhanced computed tomography findings
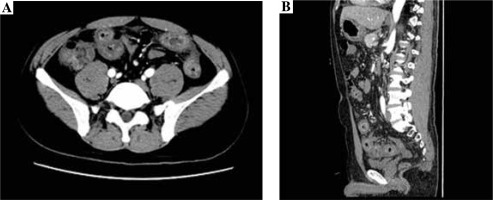
The contrast-enhanced CT examination of the abdomen showed the intestinal canal wall thickening and edema (Fig. 2).
Small intestinal endoscopy findings
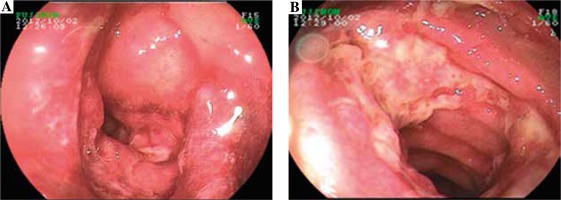
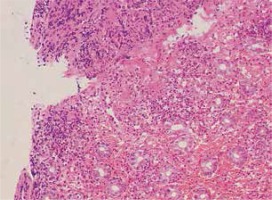
Endoscopic examination demonstrated diffused hyperemia, dropsy and erosion in the inferior segment of the ileum (Fig. 3A). And a ring-shaped ulcer was also seen in the small intestine (Fig. 3B). Pathologic examination revealed extravasation of red blood cells with mild inflammation. In addition, the examination clearly delineated a great quantity of neutrophilic leukocyte infiltration in mucosa and sub-mucosa, and obvious bleeding points in mucosa (Fig. 4).
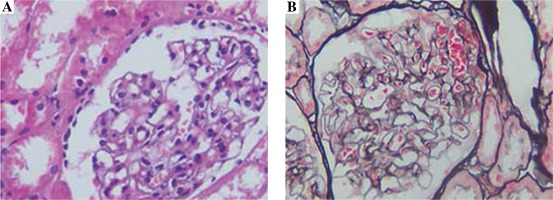
Renal needle biopsy findings
The biopsy samples obtained from a petechial area exhibited hyperplasia of mesangial cells, widening of the mesangial region and swelling of endotheliocyte (Fig. 5A). IgA deposition was observed in the mesangial region (Fig. 5B).
Immunopathogenesis of renal needle biopsy
For the 21 cases with positive urine protein, a renal needle biopsy was performed. Thirteen (61.09%) of them showed a positive staining for IgA, three (14.29%) showed a positive staining for IgA and IgG, two (9.52%) showed a positive staining for IgA and IgM, and three (14.29%) showed a positive staining for IgA+IgG+IgM. There were significant differences between patients with a positive IgA staining and patients with other positive staining proteins (p < 0.05).
Therapeutic effect
A total of 16 cases in this study were diagnosed as HSP when taken in hospital. The other 7 cases who lacked typical rash and urine protein were diagnosed as acute gastroenteritis and gastrointestinal tract bleeding. With the combined use of antihistamine drugs, gastric acid suppressants and glucocorticoids, abdominal pain and gastrointestinal tract bleeding disappeared, and urine protein decreased gradually (2.31 ±0.97 g/24 h vs. 0.63 ±0.23 g/24 h, p < 0.05). Clinical disappearance of urine protein was observed in 10/21 patients (47.6%) when they left hospital. Urine protein in the other 11 cases was still positive, but its amount decreased. Patients were advised to receive oral glucocorticoids when they left hospital.
Discussion
In the present study we have studied an unselected and large series of patients with adult mixed-type HSP that was diagnosed according to the criteria proposed by a subcommittee of the ACR, unlike other studies in which single cases were studied [20-22], so as to make this research more genuine and reliable.
In this study, we have observed that typically distributed purpuras were found in dependent areas, initially the lesions were single and little, but later they coalesced to form ecchymotic areas. The skin symptom is the most obvious and general clinical manifestation of the mixed-type HSP. So, it is the first diagnostic criterion of mixed-type HSP. But for patients with adult belly-type HSP, the main symptom is abdominal pain before the appearance of typical rash. So it was difficult to differentiate surgical abdomen from other gastrointestinal tract disease, the diagnosis for some patients was not clear even after exploratory laparotomy [23].
Therefore, we also conducted abnormal contrastenhanced CT and small intestinal enteroscopy for patients. The contrast-enhanced CT examination of the abdomen showed the intestinal canal wall thickening and edema. Endoscopic examination demonstrated diffused hyperemia, dropsy and erosion in the inferior segment of the ileum. And a ring-shaped ulcer was also seen in the small intestine. By combining the results of abnormal contrast-enhanced CT with small intestinal enteroscopy examination findings, we can diagnose the abdominal characteristics of patients.
Twenty-one (91.3%) of our adult mixed-type HSP patients were positive urine protein according to urinalysis. It was found that all the patients with positive urine protein showed significantly higher IgA levels. The IgA deposits at a glomerular capillary and mesangial region can damage small vessels, cause extensive capillaritis and arteriolitis, and immunologic injury [24]. So elevated serum IgA levels may imply involvement of immunologic elements in the pathogenesis of HSP. The finding also suggested that monitoring serum IgA levels might be useful to predict renal involvement in HSP patients.
In this study, with the combined use of antihistamine drugs, gastric acid suppressants and glucocorticoids, abdominal pain and gastrointestinal tract bleeding disappeared, and urine protein decreased gradually. Hence, this study also provided a therapy schedule for adult HSP.
Conclusions
HSP is uncommon in the adolescent age group, higher IgA levels with multiorgan involvement (gastrointestinal, kidney and skins) should make one consider the diagnosis. The combined examination of abdominal contrast-enhanced CT, small intestinal endoscopy and renal needle biopsy is a valuable method for the early diagnosis of adult mixed-type HSP.