1. Methodology of recommendation
The following paper presents the latest recommendations and the diagnostic and therapeutic guidelines on Helicabacter pylori (H. pylori) infection by the Working Group of the Polish Society of Gastroenterology. The recommendations concern: current methods of diagnosis and treating H. pylori infection in people over 18 years of age.
They include the applicability of international guidelines and available epidemiological data and are adapted for the specifics of the Polish health care system. It should be emphasised that the Polish recommendations are not a faithful copy of the European guidelines. This is due to differences in antibiotic sensitivity and the regional incidence of gastric cancer. Therefore, according to experts, it is necessary to optimise and adapt the recommendations for the population they are dedicated for.
1.1. Scope and purpose of recommendations
1.1.1. Objectives
The aim of the guideline is to improve physicians’ knowledge of the prevalence and clinical significance of H. pylori infection, to identify diagnostic methods, and to present the appropriate management of infected people according to the current state of knowledge. We expect that the application of these recommendations will translate into an increased disease diagnosis with reduced diagnosis costs, as well as an impact on the appropriate treatment of patients, especially in cases of first-line treatment failure.
1.1.2. Health issues addressed in the recommendations
The recommendations discuss in detail the health problems related to the H. pylori infection:
Have the epidemiological data on H. pylori infection changed significantly in recent years?
How does H. pylori infection occur?
What are the clinical consequences of chronic H. pylori infection?
What are the clinical manifestations (symptoms) of H. pylori infection?
When and how to test patients for H. pylori infection?
Which drugs and for how long should be used for H. pylori eradication?
How to evaluate the effectiveness of the treatment provided?
How to manage the eradication failure?
1.2. How recommendations are developed?
The source data was searched in electronic databases: PubMed, NCBI, Cochrane Library, ResearchGate, Google Scholar, as well as in guidelines published on the websites of international scientific societies (US, UK, European: AGA, ACG, USNGC, NICE, UEG).
Only original studies (optimally prospective, randomised, double-blind, controlled trials) and, in the absence of such studies: studies with a lower rank of evidence quality, up to observational and retrospective studies, excluding case series and case reports) and statistical reviews and meta-analyses were used to create recommendations. Studies published in languages other than Polish and English were excluded. Recommendations were developed in accordance with the recommendations of the Health Technology Protection Agency, the AGREE II methodology and the GRADE recommendation evaluation system were used to evaluate and describe a recommendation. Patient management questions were developed according to the PICO protocol [1, 2].
Recommendations were allocated a strength of recommendation with an additional assessment of the evidence level (discussed in Tables I and II). The method of making the final decisions involved a Delphi voting system [3]. In addition, the acceptance of each statement was discussed by a panel of experts (agreement level, Table III).
Table I
Table II
Table III
Scale determining the agreement level (rating scale) for the recommendations used in the vote [1]
| Category | Agreement level |
|---|---|
| A | Full acceptance |
| B | Acceptance with some objection |
| C | Acceptance with serious objection |
| D | Rejection with some objection |
| E | Full rejection |
Each recommendation is discussed on the basis of the scientific evidence used in its development (the link between recommendations and scientific data).
1.2.1. Interpretation of recommendations
A graphical interpretation of an example recommendation is shown below.
Each recommendation is accompanied by three information:
– the strenght of the recommendation is defined in the document as strong or weak and based on GRADE,
– quality of the evidence is defined in the document as high, moderate, weak, very weak and is based on GRADE,
– agreement level (rating scale).
Example:
4.1. Recommendation 1
For the eradication of H. pylori, we recommend the use of quadruple therapy with bismuth for 14 days in the first line of treatment. (Recommendation: strong, quality of evidence: high).
4.1.1. Voting: Agreement level ← the result of the experts’ work on the given recommendation)
A – %; B – %; C – %; D – %; E – % ← percentage of experts voting in favour of a recommendation (according to Table III).
Agreement level: ← if > 80% of experts have voted for A and B then the agreement level is high, otherwise it is low.
2. Introduction
Helicobacter pylori is a Gram-negative micro-aerophilic bacillus. To live, it requires oxygen in concentrations less than atmospheric (around 20%). In a hostile environment (low pH), it transforms into a cocci form (not a spore form but a coccoid form, in which case the bacterium does not multiply and is more difficult to damage). It primarily resides on the surface of the gastric mucosa, under the layer of mucus covering the cells, where the conditions are more favourable. It gets underneath this layer using the flagellate-covered cilia. Mucinase, produced by the bacterium, liquefies mucus, facilitating its movement. Helicobacter pylori also produces urease, an enzyme that breaks down urea to carbon dioxide and ammonia, causing the neutralisation of hydrochloric acid and an increase in pH in the immediate vicinity of the bacterium, allowing it to survive in an acidic environment. Due to outer proteins and lipopolysaccharides, it stably adheres to host cells and, thanks to its complex antioxidant system, it defends itself against attack by neutralising the oxygen free radicals produced by neutrophils. If damage to the bacterial DNA occurs, H. pylori is equipped with an efficient repair system. Vacuolising toxins and cytotoxic proteins are responsible for the actual damage to host cells. The first one (VacA) has the ability to form huge intracellular vacuoles, leading to cell damage. The expression of VacA, with varying degrees of activity, is shown by 40–60% of H. pylori strains. The activity of different alleles of VacA toxin influences its cytotoxicity, leading directly and indirectly to the initiation of apoptosis in host cells. By inducing the production of inflammatory mediators, the CagA cytotoxic protein damages the cytoskeleton, increasing further the ability of bacteria to adhere to damaged cells. The most important role of CagA is to interfere with the complex processes of cell growth and differentiation and disrupt intracellular signalling leading to cell transformation, making CagA one of the best known oncogenes of bacterial origin. To date, two subtypes of the protein have been described, 1a and 2 a. The former, East Asian, is considered more virulent, giving a stronger inflammatory response and associated with greater likelihood of malignant transformation. In Europe, including Poland, the less virulent, western subtype is predominant [4–8].
2.1. Epidemiology
The main reservoir of bacteria is human. Helicobacter pylori infection is common. It is estimated that 50% of the world’s general population is infected, although of course geographical and demographic differences are observed. A recent systematic review and meta-analysis published in Gastroenterology by Hooi et al. proved that in Europe, the prevalence of infection ranges from less than 40% of the population in Scandinavian countries and Germany, to more than 70% in Portugal. In Poland, the prevalence of infection is estimated to be between 50% and 69% with age variation [9]. Recent data published by Łaszewicz et al. in 2002–2003, showed that approximately 84% of adults and 32% of children are infected, while Szaflarska-Popławska and Soroczyńska-Wrzyszcz using a breath test found infection in 23.6% of young people aged 13–17 years (data for 2008–2015) [10, 11].
Infection usually occurs in childhood or adolescence via oral route. It is believed that the infection is usually lifelong. Although the dominant belief is that transmission is most often from mother to child (or within the family), there are no studies of sufficient quality to justify, for example, family eradication or prevention of infection [12].
2.2. Spectrum of symptoms and complications of infection
The different virulence of different proteins and toxins from different strains translates into a different clinical picture of infection. An overwhelming proportion (up to 80%) of infected people have no symptoms or, importantly, no disease complications. Also, there are no pathognomonic signs of infection. The most common diseases that may or may not be associated with H. pylori infection include:
a) Dyspepsia
Dyspepsia is defined as epigastric pain, usually postprandial or meal-related, early satiety, postprandial fullness. It affects up to 20% of the general population. There are many reasons for the phenomenon, and in countries with H. pylori infection rates > 15% of the population, the infection is thought to be such a common cause of symptoms (20–50% of dyspepsia patients) that every patient requires its exclusion [13].
b) Gastritis
Generally, H. pylori always causes an acute phase of inflammation by damaging the gastric mucosa. However, most often (in about 80% of cases) there are no clear, acute or chronic disease symptoms. It is known that most often the infection progresses to the chronic phase, which is latent in up to 90% of patients. Only 10–20% of people are symptomatic, due to inflammation in the pre-pyloric stomach area, gastrin overproduction and the development of ulcers area (due to its separation from the parietal cells). Approximately 5% of people develop extensive atrophic inflammation. It is a particular form of inflammation that significantly increases the risk of gastric cancer development based on cell atrophy and the occurrence of intestinal metaplasia followed by dysplasia (Correa’s cascade) [14, 15].
c) Peptic ulcer disease
An ulcer, i.e. a full-thickness defect in the mucosa, exceeding the muscle layer and extending to the submucosa or even deeper, is the consequence of an imbalance between irritants (hydrochloric acid, pepsin) and protective factors (a properly vascularized and mucus-covered mucosa), the production and function of which is affected by infection. The H. pylori infection is responsible for about 75–90% of duodenal ulcers and about 70% of gastric ulcers. It results in hypergastrinemia, excessive production of hydrochloric acid, damage to the mucus layer and mucosa (development of a local inflammatory response) and, finally, weakened defence processes. All this leads to the development of ulcers [5, 14].
d) Stomach cancer
Thanks to numerous epidemiological, molecular and animal studies and a wealth of data on the reduction of gastric cancer incidence in populations undergoing eradication of H. pylori infection, it is now recognised as a major aetiological factor of gastric cancer. It is estimated that up to 90% of cases may be caused by infection, which is considered both intestinal and diffused types [16, 17].
e) Gastric MALT lymphoma
Long-term immune system stimulation due to infection is capable of leading to uncontrolled monoclonal proliferation and the development of gastric MALT lymphoma. Approximately 90% of these arise from infection (particularly with high VacA virulence strains) and, interestingly, the first line of therapy for malignant tumours is the treatment of H. pylori infection.
f) Other diseases
Helicobacter pylori infection is associated with many other diseases of different organs and systems. Although many of these relationships still remain hypothetical, a correlation between infection and spontaneous thrombocytopenic purpura, iron and/or vitamin B12 deficiency anaemia, as well as between certain cancers (e.g. hepatocellular carcinoma), myocardial infarction or asthma has been proven to date. In 2020, the first review of meta-analyses and systematic reviews on the association between H. pylori infection and various diseases was published in the British Medical Journal. 88 publications were meticulously analysed (out of a baseline of more than 3,000 considered), discussing both the harmful and protective role of infection. Those areas where controversy still exists (e.g. cardiology) were analysed [17]. Most such associations have been proven in Asian studies, but there is also good evidence of the association between the infection and diseases of various systems (such as cancer, diabetes, cirrhosis, Parkinson’s disease, myocardial infarction, COPD or glaucoma), in Europe and in the Western countries.
3. Indications for Helicobacter pylori testing
3.1. Statement 1
| Gastritis caused by Helicobacter pylori is infectious disease regardless of the symptoms and complications. |
| Quality of evidence: high. Recommendation: not applicable. |
3.1.2. Discussion
Helicobacter pylori cannot be considered as a commensal microbiota, as its presence in the stomach induces inflammatory response of varying severity irrespective of the presence of ailments [18].
Indeed, the spectrum of symptoms and disease consequences varies between patients and ranges from asymptomatic forms to peptic ulcer disease and gastric cancer, most often untreatable at the time of diagnosis. Inflammation is caused by multiple mechanisms, induced both in gastric epithelial cells, which are the site of first contact with the pathogen, and in circulating immune cells recruited to the site of infection. Helicobacter pylori stimulates the production of pro-inflammatory cytokines such as: IL-1, IL-6, IL-8, TNF and RANTES [19]. On microscopic examination, chronic active inflammation with infiltration of neutrophils and mononuclear cells is observed. It has been shown that the cure of H. pylori infection leads to withdrawal of inflammatory lesions and restoration of normal mucosa, provided irreversible changes have not occurred. It is thought that some forms of mucosal atrophy may even withdraw [20].
3.2. Statement 2
3.2.2. Discussion
In real world, it is most important to distinguish between indications for the diagnosis of infection and indications for its eradication. Until recently, tests were only performed for clinical indications (disease symptoms) and treatment was recommended for all those found to be infected. Several guidelines with the status of international recommendations have emerged between 2014 and 2022, including a report by the International Agency for Research on Cancer (IARC), the WHO opinion, the so-called Kyoto consensus or the Maastricht recommendations (fifth and sixth editions), which include world-known experts in the field among their authors, in which the approach to mass testing and eradication is changing [16, 21, 22]. While in countries with a high risk of gastric cancer, it should be recommended, for countries with an average or low incidence (including Poland), unequivocal evidence for the validity of such a procedure is lacking. Helicobacter pylori infection is necessary but not sufficient for the development of gastric cancer. In addition, the infection has a protective effect in isolated but civilisation-relevant diseases such as childhood obesity and asthma. In the US, there are even stricter recommendations, issued in 2017, where testing is not offered to patients with vitamin B12 deficiency, with a family history of gastric cancer or with hyperplastic polyps [23].
At stake in mass eradication, as mentioned, is a reduction in the incidence of gastric cancer (46–51% reduction in Asian populations). In 2020, the results of two studies prospectively evaluating the effectiveness of eradication were published. A study published in Gut involving more than 85% of the population of the Matsu Islands (China), in which the incidence of post-interventional infection decreased from 64.2% to 15% between 2004 and 2018, showed that the incidence of gastric cancer decreased by 53%. In a prospective randomised, placebo-controlled study evaluating the effectiveness of H. pylori eradication in first-degree relatives of people with gastric cancer, from South Korea (New England Journal of Medicine), eradication was shown to significantly reduce the risk of cancer (by 45%) [24, 25]. The evidence for reductions in incidence and mortality does not only extend to high-risk countries. In an US retrospective cohort study, the effectiveness of eradication in the prevention of gastric cancer was proven, but again, the greatest benefit was for non-white races. A Markov model developed in this country to assess the cost and effectiveness of gastric cancer screening confirmed these observations. Screening and surveillance for abnormalities was only cost-effective in black, Latin American and Asian individuals.
It should therefore be considered that, under Polish conditions, patients with symptoms or diseases with a proven link to infection should be tested (Table IV) and, most likely, all patients undergoing gastroscopy (most indications include diseases that may be related, even indirectly, to infection, although further studies are needed to support this hypothesis). If infection due to other causes is detected, the decision to eradicate should be taken individually after discussion with the patient.
Table IV
Indications for testing and eradication of H. pylori – a summary
At this stage, there is insufficient evidence for testing the general population in Poland and Europe [24–28].
3.3. Statement 3
3.3.2. Discussion
The H. pylori infection is a potentially curable cause of dyspepsia, peptic ulcer disease or gastric cancer. Indications for endoscopic diagnosis of infection vary according to the risk of malignancy and previous therapies and include older age (> 50 yrs), male sex, the presence of gastric cancer in first-degree relatives, a pepsinogen I/III ratio < 3 or smoking [22, 29]. This means that the majority of subjects with a suspected infection have an indication for gastroscopy. On the other hand, the main indications for gastroscopy are epigastric pain, dyspepsia, nausea and vomiting, anaemia and overt gastrointestinal bleeding, followed by reflux disease and cirrhosis surveillance. Thus, in more than 80% of indications for gastroscopy, there are, by definition, indications for a test for H. pylori. Despite the lack of formal studies, many recommendations consider gastroscopy to be generally associated with testing for infection and, according to European experts, the procedure is incomplete without a test [30]. Due to the possibility of false-negative results during treatment with proton pump inhibitors (PPIs), the indications for testing during therapy should be determined on an individual basis.
3.4. Statement 4
| In the diagnosis of undiagnosed or functional dyspepsia, first H. pylori infection should be excluded. |
| Quality of evidence: high. Recommendation: strong. |
3.4.2. Discussion
A ‘test and treat’ strategy based on non-invasive diagnostic testing for H. pylori infection, and treatment in the event of a positive result, in countries with high rates of H. pylori infection is considered a safe and cost-effective strategy for managing patients with symptoms of undiagnosed dyspepsia [31, 32]. A detailed discussion on the management of dyspepsia is the subject of separate recommendations and is beyond the scope of this publication. However, it should be emphasised that more than a dozen large, good-quality prospective randomised controlled studies have confirmed the validity of such a management. A 2019 network meta-analysis by Eusebi et al. which included 15 randomised trials (6,162 patients), showed that the strategy in question was the best among all competing management strategies. It significantly reduces the need for gastroscopy (23% of patients in this group required endoscopy) [33]. In simulation modelling of different strategies, Beresniak et al. proved that the ‘test and treat’ strategy is twice as cheap as an endoscopy-based strategy and 12.5% cheaper than a symptomatic treatment strategy (treat symptoms first, test for H. pylori later) [34].
3.5. Statement 5
3.6. Statement 6
3.6.2. Discussion
Discussion of statements 5 and 6.
In clinical practice for the diagnosis of dyspepsia, we can use non-invasive tests for the detection of H. pylori infection with proven and acceptable effectiveness. The need for the test alone is not an indication for gastroscopy. Depending on the geographical region, the prevalence of gastric cancer in the population and the epidemiology of other risk factors for organic upper gastrointestinal disease, the age at which endoscopic examination is recommended for patients with dyspepsia symptoms ranges from 45 to 60 years [22, 31–34].
Poland is one of the countries with a low incidence of gastric cancer. According to the 2019 National Cancer Registry (NCR), 5,100 new cases were detected in Poland (standardised incidence rate – 22/100,000), and year-on-year the incidence is decreasing [35]. Still, gastric cancer is diagnosed at advanced stages, when treatment options are limited. Recent Polish data from a nationwide study by Januszewicz et al. showed that up to 6% of gastric cancers are missed (undiagnosed) during the first diagnostic gastroscopy [36]. Although the mean age at diagnosis of cancer in this study was about 68 years, and the incidence is known to increase with age, KRN data indicate that the first noticeable increase in gastric cancer incidence is observed from 45 years of age onwards both in males and females [35]. These data are consistent with recent large population-based studies published in 2022 in Nature and Lancet analysing trends in gastric cancer incidence and mortality from the ‘90s to estimated figures in 2040. An increase in incidence is seen from 45 years of age onwards, particularly in females (the variation in age groups is not as clear) [37, 38]. Therefore, given the lack of population-based screening in Poland, the prevalence of H. pylori infection and the high prevalence of other risk factors for gastric cancer in patients with upper gastrointestinal symptoms, despite the lack of scientific studies, we maintain our previous recommendation to perform gastroscopy after 45 years of age [39, 40].
3.7. Statement 7
| Suspicion and/or diagnosis of atrophic gastritis is an indication for diagnosis of H. pylori infection. |
| Quality of evidence: high. Recommendation: strong. |
3.7.2. Discussion
Helicobacter pylori infection is a predisposing factor for the development of chronic atrophic gastritis. This, in turn, is a recognised risk factor for gastric cancer [7, 14]. It has been shown that eradication of the infection is most effective in terms of cancer prevention if carried out before chronic atrophic inflammation develops [41]. Even when atrophy occurs, eradication can reverse the process and, at least to some extent, also stop the progression from intestinal metaplasia into neoplastic lesions [42]. For a detailed discussion of the relationship between H. pylori and cancers arising from atrophic gastritis, see ‘Prevention and public health’.
3.8. Statement 8
| The indication for the diagnosis of H. pylori infection is gastric and/or duodenal ulcer disease – active, inactive and complicated. |
| Quality of evidence: high. Recommendation: strong. |
3.8.2. Discussion
The first references to a potentially pathogenic bacterium detected in mammalian stomachs appeared as early as the end of the 19th century in Germany, Italy and Poland (Walery Jaworowski, 1899) [43]. However, it was not until the 1980s that B.J Marshall and J.R. Warren proved the link between Helicobacter infection and gastritis symptoms, for which they were awarded the Nobel Prize in Medicine in 2005, it became clear that the aetiology of peptic ulcer disease is overwhelmingly infectious and contagious [44]. The research from the late second decade of the 21st century confirms the epidemiology of gastric and duodenal ulcer disease. It is estimated that around 70–80% of gastric ulcers and up to 90% of duodenal ulcers are caused by infection. Eradication of H. pylori reduces the risk of their development and, if they do occur, shortens healing time and reduces the risk of recurrence [45, 46].
3.9. Statement 9
3.9.2. Discussion
Helicobacter pylori is a recognised risk factor for gastric cancer and lymphoma. Detailed relationships between infection and carcinogenesis are presented in the discussion of steatment 2 and in the chapters ‘Introduction’, ‘Prevention’ and ‘Public health’. In this discussion, it is worth citing the results of three good-quality studies published in the New England Journal of Medicine, Gut and Gastroenterology. The first study, by Choi et al. 2020, looked at first-degree relatives of gastric cancer patients [25]. In this single-centre, double-blind, placebo-controlled study, H. pylori-infected relatives were subjected to eradication or followed up (after placebo administration). After a mean follow-up of 9.2 years, eradication was shown to more than double (HR = 0.45, p = 0.03) the risk of gastric cancer. Furthermore, half of the cancer patients were found to have a chronic infection. In a study, Chiang et al. assessed the impact of mass eradication on gastric cancer incidence and mortality [24]. Eradication began throughout the study area in 2004 and continued until 2018. During this time, six rounds of eradication took place. Again, this study showed a reduction of more than half (53% reduction, p < 0.001) in cancer incidence compared to pre-eradication data. The mortality rates have decreased by 25%. The most recent study, Yan et al. 2022, was an update after more than 26 years of a study evaluating the effect of eradication on gastric cancer prevention [47]. Patients were originally subjected to eradication in 1994 and their fate was now analysed after several decades of regular follow-up and endoscopic surveillance. The eradication group had a 43% lower risk of cancer, and when those without pre-cancerous lesions on index gastroscopy were assessed, the risk was lower by 63%. These findings are consistent with previous research.
3.10. Statement 10
3.10.2. Discussion
Non-steroidal anti-inflammatory drugs (NSAIDs) including aspirin (ASA) still are an independent risk factors for peptic ulcer disease and its complications [48]. Some studies have also shown that patients with H. pylori infection using NSAIDs have a higher risk of developing the disease than uninfected patients, and the risk of ulcer bleeding is even several times higher in these patients [49]. The greatest benefit of eradication was shown in the group of patients prior to starting long-term NSAIDs treatment, whereas in those already treated, the risk of developing peptic ulcer disease was comparable to that of patients not subjected to eradication [50]. Nevertheless, in recent years, eradication has also been shown to reduce the risk of ulcer recurrence in NSAIDs-treated patients [51]. Therefore, eradication is indicated in patients with a history of gastric and duodenal ulcers and using NSAIDs. In 2022, the results of the HEAT study, which evaluated the effect of H. pylori eradication in the primary prevention of upper gastrointestinal ulcer bleeding, were published in Lancet [52]. This was a prospective, randomised, placebo-controlled study in patients over 60 years of age. Eradication has been shown to reduce the risk of bleeding by 2.5 times in the first 2.5 years post eradication, and then this effect disappears. This is the first observation of its kind, shedding completely new light on the seemingly familiar and certain issue of reducing the risk of ulcer complications through H. pylori eradication. This may partly explain the observations of clinicians who encounter a high number of complications in daily practice, although of course the findings need to be confirmed.
Antiplatelets other than aspirin are unlikely to increase the risk of ulcer bleeding in patients without H. pylori infection, whereas the risk of ulcer bleeding in patients with H. pylori infection was significantly increased in patients on combination therapy: ASA + NSAID (OR = 5.85). Thus, prior to the initiation of anticoagulant and/or antiplatelet treatment, as a general rule the diagnosis for H. pylori and prior eradication should always be considered.
It should also be mentioned that there is growing evidence of adverse effects of serotonin reuptake inhibitors on the gastrointestinal mucosa manifested by peptic ulcer disease and bleeding, particularly in patients already taking NSAIDs. In 2022, a meta-analysis on this question was published in Nature and proved that the risk of bleeding was 75% higher in the group using drugs from both classes concomitantly [53].
3.11. Statement 11
3.11.2. Discussion
In 2020 The American Gastroenterology Association (AGA) has published recommendations for the management of iron deficiency anaemia, in which, based on the available scientific evidence, it recommends the early H. pylori eradication as a potential reason [54]. Meanwhile, the UK guidance (BSG) issued in 2021 mentions this possibility, but makes it clear that the data are limited and/or contradictory [55]. However, it is worth noting that we have results from studies showing that iron supplementation in patients with iron deficiency anaemia and H. pylori infection is effective, while combination therapy (iron supplementation and eradication) is statistically significantly better.
We do not have good quality studies linking eradication to the resolution of vitamin B12 deficiency anaemia. However, given the potential mechanism of its formation (atrophy due to infection, resulting in impaired absorption), it is worth considering eradication in this group of patients.
In adults with idiopathic thrombocytopenic purpura, H. pylori eradication gives a sustained improvement by increasing platelet counts, as shown in a systematic review of 25 studies published in Blood [56]. Also, haematology recommendations recommend testing and, if infection is confirmed, treatment of H. pylori infection as part of the management of thrombocytopenic purpura [57].
3.12. Statement 12
3.12.2. Discussion
Helicobacter pylori infection causes not only macroscopic but also microscopic duodenitis. If biopsy samples need to be taken, in the differential diagnosis of diseases with an inflammatory infiltrate (visceral disease, Crohn’s disease, etc.), sections should be taken for urease testing concomitantly with taking sections for histopathological examination [58].
3.13. Statement 13
| There is insufficient evidence of a correlation between the occurrence of hyperplastic polyps or lymphocytic inflammation and H. pylori infection. |
| Quality of evidence: very low. Recommendation: weak. |
3.13.2. Discussion
Several retrospective observational studies have suggested a possible relationship between the development of hyperplastic polyps and H. pylori infection. However, to date there is insufficient evidence to support this relationship, and recent work even suggests that the incidence of hyperplastic polyps is increasing, even though the incidence of H. pylori infection is decreasing [59]. There is even less data linking lymphocytic gastritis to infection, and in 2014 Nielsen et al. published the results of a study in which this relationship was not confirmed [60].
3.14. Statement 14
| After completion of treatment for H. pylori infection, it is advisable to assess the treatment effectiveness. |
| Quality of evidence: low. Recommendation: weak. |
3.14.2. Discussion
For a detailed discussion of this question, see statement 24. This statement was kept for consistency in the recommendation and discussion of indications for testing for H. pylori infection. Due to the prevalence of the infection and the potential for increasing bacterial resistance to the eradication regimens used, it is advisable to monitor the treatment effectiveness. Patients who have not been cured have an increasing risk of complications. The common availability of cheap and effective diagnostic tests makes it easy to monitor the therapeutic effect. To date, there is no good scientific evidence of the cost-effectiveness of such management (with the exception of bleeding ulcers, which used to be one of the few indications for cure assessment). The need to maintain the optimal eradication regimen in a specific population also supports the assessment of treatment effectiveness, which would not be possible without systematic confirmation of its effectiveness.
3.15. Statement 15
3.15.2. Discussion
As mentioned before, no benefit for mass screening and eradication has been demonstrated in countries with a low risk of gastric cancer. More information can be found in the ‘Introduction’ section and in the ‘Prevention and Public Health’ chapter. However, it should be remembered that Poland is one of the countries with a low and decreasing risk of gastric cancer. No epidemiological studies have yet been carried out to estimate the dependence of this trend on the introduction of universal testing and treatment of infection. In countries with a high risk of gastric cancer, the evidence for the effectiveness of eradication in preventing gastric cancer is growing, and it is strong enough that, in general, screening is taking effect. In mid-November 2022, the WHO International Agency for Research on Cancer (IARC) launched a new pan-European project to accelerate the reduction of gastric cancer in Europe through the eradication of H. pylori infection: EUROHELICAN). The project co-funded by the European Union and is intended to introduce population-based screening (‘test and treat’ strategy) to prevent the development of gastric cancer in Europe [61]. A multicentre study has also been initiated in European countries with regions at highest risk of gastric cancer, which is designed to evaluate the effect of H. pylori eradication on cancer mortality in people between 40 and 64 years of age (GISTAR study) [62]. The study is planned to include 30,000 participants and results can be expected after 15 years.
4. Diagnostic methods
4.1. Statement 16
4.1.2. Discussion
Diagnosis of H. pylori infection is recommended only for planned treatment. It is not recommended to start eradication therapy without confirmation of current infection by suitably reliable methods [22, 23]. The available diagnostic methods can be classified as invasive and non-invasive.
For all gastroscopy-based invasive tests and non-invasive tests that confirm active H. pylori infection (faecal H. pylori antigens and breath test) proton pump inhibitors (PPIs) should be discontinued for 14 days prior to testing, and bismuth and antibiotics should be discontinued for 4 weeks prior to testing [22, 34, 39, 63]. Failure to follow these recommendations may reduce the sensitivity of the tests performed.
The choice of diagnostic method depends on the patient’s current clinical situation and the need for gastroscopy [22, 63, 64]. If there is no indication for gastroscopy then a test to detect H. pylori antigens in faeces or a breath test is the recommended exam [22]. Helicobacter pylori antigens in faeces are detected using immunochemical methods – immunoenzymatic and immunochemiluminescent test using monoclonal antibodies and automated analysers [64]. The so-called rapid tests, requiring no measurement device, based on immunochromatographic methods with monoclonal or polyclonal antibodies are also available – these are not recommended due to their variable diagnostic accuracy [64]. The detection of H. pylori antigen in faeces is indicative of ongoing infection. Faecal antigen testing can therefore be used to establish an initial diagnosis of H. pylori and to confirm eradication [64, 65]. Of the available tests, faecal antigen testing is most cost-effective in regions with low to moderate H. pylori prevalence [65]. Active H. pylori infection in the stomach is manifested by the presence of bacterial antigens in faeces. Many tests can detect H. pylori-specific antigens (e.g. catalase) in faeces, providing a convenient, non-invasive diagnostic tool [66, 67]. Newer tests using monoclonal antibodies perform better in comparative studies [68–70]. The sensitivity and specificity of the laboratory immunoassay using monoclonal antibodies (94% and 97%) are comparable to the urea breath test (UBT) [70–72]. In cases of active bleeding in peptic ulcer disease, the specificity of H. pylori faecal antigen testing may be reduced. However, the sensitivity of the immunoassay with monoclonal antibodies remains high in cases of recent bleeding due to peptic ulcer disease [73]. Faeces antigen testing using a polyclonal enzyme immunoassay is no longer used due to its low sensitivity. Rapid, in-office monoclonal immunochromatographic tests for faecal antigens have high specificity, but their use has been limited by low sensitivity (96 and 50%, respectively) [74].
The urea breath test is a highly researched and widely recommended non-invasive test as part of an ‘test and treat’ strategy [22]. This test is based on the hydrolysis of urea by H. pylori urease to NH3 and CO2. The patient ingests urea labelled with non-radioactive 13C (preferred) or radioactive 14C (rarely used; radiation dose is minimal [approximately 1 µCi], contraindicated in children and pregnant women) [64]. The resulting 13CO2 or 14CO2 is expelled through the lungs with the expired air. An increase in 13CO2 concentration after urea ingestion relative to baseline or a positive 14CO2 assay (undetectable under normal conditions) indicate urease activity in the stomach, indicating the presence of H. pylori). The 13C-UBT test has a high diagnostic value, as it has a sensitivity of 88–95% and a specificity of 95–100% [75, 76]. False-positive results are rare. False-negative results can be observed, among others, in patients taking PPIs, bismuth or antibiotics and in the situation of active bleeding from a peptic ulcer [22, 64]. To improve the diagnostic value of UBT, the use of citric acid in the test meal is suggested. Citric acid helps to slow gastric emptying and improves the distribution of gastric contents by increasing the contact time with H. pylori urease [22, 76]. Whether H2 blockers affect the sensitivity of UBT is controversial [77].
4.2. Statement 17
4.2.2. Discussion
The sensitivity and specificity of IgG antibody assays in detecting H. pylori infection is low (approximately 80%) [22, 39, 64]. With simultaneous IgG and IgA assays, the diagnostic value increases slightly. Serological tests require validation at the local level, as H. pylori strains are diverse and it is virtually impossible to achieve it in routine practice [22]. Guidelines recommend that serological tests should not be used in low-prevalence populations, as their low diagnostic value may result in inappropriate treatment in a significant number of patients [22, 39].
A positive result is not indicative of current infection, as the presence of antibodies is observed for many months after cure. For this reason, serological tests are not suitable for the assessment of effectiveness of eradication. Serum IgG against H. pylori assays can serve as a screening test in specific clinical situations: a) during treatment with PPIs and antibiotics, b) in patients with other factors reducing the sensitivity of the other tests, e.g. active or recent gastric ulcer bleeding, atrophic gastritis or cancer (if the patient has not received prior eradication treatment) [22, 64]. The determination of antibodies against H. pylori can be used for screening when other diagnostic methods are unavailable or upper gastrointestinal endoscopy cannot be performed [22, 39]. In the event of a positive serology test, infection should be confirmed using another method (faecal H. pylori antigens or urea breath test) before eradication treatment is introduced.
4.3. Statement 18
4.3.2. Discussion
Histological examination of gastric mucosal sections is a commonly used method with a high diagnostic value (sensitivity 95%, specificity 98%) [78]. The section is stained with haematoxylin and eosin and additionally with special stains (e.g. Giemsa or Warthin and Starry) [78]. Most H. pylori infections can be diagnosed using a histological examination with histochemical staining alone. In cases of chronic (active) gastritis in which H. pylori bacteria is not found by histochemical staining, an immunohistochemical study may additionally be performed. If the image of the examined section is normal, there is no need to deepen the diagnosis. Histological examination also makes it possible to assess lesions in the gastric mucosa. For the assessment of gastritis caused by H. pylori, at least 2 sections should be taken during gastroscopy from the pre-pyloric part of the stomach (from the greater and lesser curvature 3 cm proximal to the pyloric duct) and 2 sections from the middle part of the gastric body [22]. Consideration should also be given to taking additional sections from the angular notch to detect precancerous lesions. The sections should be put in separate containers. If gastric sections are taken correctly (according to the guidelines), it is not necessary to perform a urease test. The sensitivity of histological examination may be reduced in patients with bleeding ulcers and in patients using PPIs, due to migration of H. pylori into the proximal part of the gastric body [79] (Table V).
Table V
Diagnostic methods used in the diagnosis of H. pylori infection
5. Treatment
5.1. Statement 19
5.1.2. Discussion
The consensus of experts is that optimal H. pylori eradication treatment should be effective from the first treatment attempt and have at least 90% success rate [22, 23, 80]. Consequences of ineffective eradication include clinical complications associated with persistent H. pylori infection, repeated exposures to antibiotics and gastric acid inhibitors, generation of antibiotic resistance among the bacteria, and increased direct and indirect healthcare costs. The most important factors responsible for treatment failure are bacterial resistance to antibiotics and non-compliance. Therefore, the doctor should carefully discuss the treatment regimen, the benefits of eradication and possible side effects with the patient before prescribing the therapy. The results of the European Registry on H. pylori management (Hp-EuReg) confirmed that patient’s compliance significantly increased the effectiveness of eradication (87%), compared with non-compliance (56%) [81]. The patient should be warned about the adverse effects of smoking on the treatment effectiveness [82]. Smoking increases gastric acid secretion, impairs mucus secretion and reduces submucosal blood flow in the stomach, thereby reducing the local penetration of antibiotics.
Current European guidelines suggest determining the sensitivity of H. pylori to antibiotics (culture, molecular testing) even before using first-line therapy [22]. If the antibiotic sensitivity profile of H. pylori is unknown (as in the vast majority of cases), a thorough history of the patient’s previous exposure to antibiotics should be taken before prescribing eradication treatment. This recommendation is particularly relevant for further attempts to use clarithromycin and levofloxacin, as resistance rates to clarithromycin have been shown to reach 15–67% and 19–30% to levofloxacin in these situations [83]. A survey for the eradication treatment prescribers showed that only 38% had collected information from patients regarding previously used antibiotics [84].
5.2. Statement 20
Table VI
First-line treatment in H. pylori eradication
5.2.2. Discussion
The increasing resistance of H. pylori to antibiotics is the reason for constantly changing treatment regimes. The World Health Organization (WHO) has identified H. pylori as one of the 12 highest priority pathogens for the development of new, effective antibiotics [85]. In a meta-analysis of 50,000 patients from 45 countries, primary resistance to clarithromycin ranged from 10% to 34% and to metronidazole from 23% to 56% [83]. In a Polish study of paediatric and adult populations, H. pylori resistance to clarithromycin was 28% and to metronidazole 46%, with combined resistance to both antibiotics as high as 20% [86]. The analysis of the population of south-western Poland confirmed resistance to clarithromycin and metronidazole of 46% and 56% respectively [87]. The use of triple therapy with clarithromycin, in the case of bacterial resistance to this antibiotic, resulted in a very low cure rate of only 43% [88]. Therefore, classic triple therapy (PPI + clarithromycin + amoxicillin or metronidazole), should not be used in Poland currently.
Quadruple therapy with bismuth has high effectiveness and reduces the issue of clarithromycin resistance [89–91]. Resistance to metronidazole can be overcome by acting synergistically with bismuth and increasing its daily dose to 1.5–2 g [92]. An analysis of 21,533 patients from the Hp-EuReg registry showed that quadruple therapy with bismuth had over 90% effectiveness in the first-line eradication treatment [93]. The use of this treatment in regions with high clarithromycin resistance had no effect on the high percentage of successful eradication – 94.4% [91]. A multicentre, prospective study with 2,100 patients with H. pylori infection showed the effectiveness of quadruple therapy with bismuth in first-, second- and subsequent-lines to be 95%, 89% and 92%, respectively, based on the modified intention-to-treat (m-ITT) analysis [94]. Two bismuth-containing preparations are currently available in Poland: Pylera (a combination drug, one capsule contains 140 mg of bismuth tripotassium dicitrate, i.e. 40 mg of bismuth oxide, 125 mg of metronidazole and 125 mg of tetracycline hydrochloride), and Ulcamed, which contains bismuth only (one tablet contains 120 mg of bismuth oxide as bismuth tripotassium dicitrate).
In the absence of availability or intolerance of bismuth preparations, an alternative form of first-line treatment is bismuth-free quadruple therapy, with a high eradication success rate of more than 90% [93]. It is important to remember that if there is a high (> 15%) proportion of H. pylori strains resistant to both clarithromycin and metronidazole in a given area, the effectiveness of quadruple therapy without bismuth is significantly lower, i.e. 79% [95]. Despite initially encouraging results, sequential or hybrid treatments are currently not recommended for the treatment of H. pylori infection due to their complicated administration and lower effectiveness than bismuth-free quadruple therapy [22, 80, 95].
5.3. Statement 21
| The recommended duration of quadruple therapy with bismuth is 14 days. The recommended durations of bismuth-free quadruple therapy is 14 days. |
| Quality of evidence: very low. Recommendation: weak. |
5.3.2. Discussion
The results of a meta-analysis evaluating the effectiveness, safety and compliance rates showed ≥ 85% effectiveness of quadruple therapy with bismuth administered for 10–14 days, compared to a shorter treatment duration [96]. Given the high prevalence of metronidazole resistance in many countries, also in Poland, the recommended eradication period is 14 days [22, 80]. Alternatively, quadruple therapy with bismuth for 10 days can be used if its effectiveness has been confirmed in the given region [22]. A meta-analysis of 30 studies involving nearly 6,500 patients confirmed the high, 90% effectiveness of 10-day treatment using a combination bismuth preparation (Pylera) in an intention-to-treat (ITT) analysis, regardless of dose or type of PPI [89]. The results of the Hp-EuReg registry, reflecting the real-world data, showed the effectiveness of 10-day therapy in first-, second- and subsequent lines treatment to be 94%, 90% and 86%, respectively [94]. In a retrospective analysis in north-eastern Poland, the effectiveness of 10-day treatment using a combined bismuth preparation was 89.4% [97]. Currently, there are no studies directly comparing classic quadruple therapy with bismuth with a combination preparation for 10 or 14 days.
For bismuth-free quadruple therapy, the recommended treatment duration is 14 days [22]. A real-life study conducted in Italy found a statistically significant superiority for 14-day therapy over 10-day therapy (ITT 96.1% vs. 80%, p = 0.001) [98]. In an analysis of 4,164 patients from the Hp-EuReg registry treated empirically with bismuth-free quadruple therapy in the first-line setting, a 14-day eradication superiority was demonstrated over 10-day eradication (m-ITT 92.1% vs. 88.3%) [93].
Helicabacter pylori is most sensitive to antibiotics when the stomach pH remains between 6 and 8, as this is the optimal environment for bacterial replication. In addition to the bactericidal effect found in vitro, proton pump inhibitors, by increasing gastric pH, enhance the penetration of antibiotics into the mucosa and increase their stability and antimicrobial activity. The level of inhibition of gastric acid secretion by PPIs is strongly dependent on the patient’s ability to metabolise the drug, which is determined by the polymorphism of cytochrome 2C19 (CYP2C19). In individuals with the CYP2C19 genetic polymorphism who metabolise drugs rapidly (56–81% of the Caucasian population), the use of older-generation PPIs (omeprazole, lansoprazole), which are mainly utilised by this cytochrome, is associated with a higher rate of eradication failure [99]. A meta-analysis evaluating the effect of PPIs on the effectiveness of 3-drug therapy with clarithromycin showed that the use of high versus standard doses of PPIs, improved eradication rates by 8% (82% vs. 74%, RR = 1.09, 95% CI: 1.01–1.17) [100]. This effect was particularly obvious when esomeprazole 40 mg was compared to omeprazole (20 mg) or pantoprazole (40 mg) – an 11% improvement in eradication effectiveness. Quadruple therapy with bismuth showed no difference in treatment effectiveness between low, standard and high doses of PPIs [16]. In contrast, the superiority of high over low doses of PPIs was found for quadruple therapy without bismuth [93].
Vonoprazan is a drug that inhibits the ATP-H+/K+ pump in gastric lining cells in a competitive, potassium-dependent manner. Compared to PPIs, the effect of vonoprazan is more rapid, lasts longer, produces a stronger inhibition of gastric acid secretion, and is independent of food intake and CYP2C19 polymorphism [101]. In a meta-analysis of trials on triple therapy with clarithromycin, replacing PPIs with vonoprazan increased eradication effectiveness by 20% [102]. Preliminary results also indicate a high, 90-95% effectiveness of vonoprazan in dual therapy with amoxicillin (as first and second-line treatment) [103].
5.4. Statement 22
5.4.2. Discussion
Determining the antibiotic sensitivity profile of H. pylori enables the implementation of targeted treatment and prevents the build-up of antibiotic resistance. As obtaining this information requires, in most cases, the collection of microbiological material during endoscopy, such management is rare in everyday clinical practice. If the antibiotic sensitivity profile of H. pylori is not known, the type of second-line treatment should be made on the basis of previous antibiotic exposure or the known regional prevalence of resistance of the micro-organism. There are no literature data to support the superiority of targeted antibiotic therapy over empirical antibiotic therapy in patients after one or more unsuccessful eradication attempts [104, 105]. A meta-analysis of 13 studies (mostly second-line treatment) showed similar effectiveness of targeted and empirical therapy (RR = 1.09; 95% CI: 0.97 to 1.22) [106]. Close surveillance of the effectiveness of eradication and monitoring of local antibiotic resistance rates are crucial in choosing the optimal empirical therapy.
If quadruple therapy with bismuth fails as first-line treatment, the next regimen should include triple therapy with levofloxacin or double therapy with high doses of PPI and amoxicillin. A literature review with meta-analysis of 54 randomised clinical trials showed that levofloxacin used for a minimum of 10 days in triple therapy (PPI, levofloxacin, amoxicillin) or quadruple therapy (PPI, levofloxacin, bismuth, amoxicillin or tetracycline) was superior to quadruple therapy with bismuth in second-line settings [107]. When prescribing levofloxacin-containing eradication therapy, it is important to take into account the prevalence of resistance to this antibiotic, which is found in up to 31% of infected patients in the United States and 14% in Poland [108, 109]. The addition of bismuth to triple therapy with levofloxacin has been shown to have a beneficial effect. Comparing levofloxacin therapy (PPI, amoxicillin, levofloxacin) with and without bismuth showed a slight superiority for bismuth therapy (87% vs. 83%), particularly obvious in the case of concurrent levofloxacin resistance (71% vs. 37%) [110]. In patients following an unsuccessful eradication attempt, the use of quadruple therapy with bismuth, levofloxacin, amoxicillin and esomeprazole for 14 days showed a high effectiveness of 90% [111].
As H. pylori resistance to amoxicillin is occasional, using dual therapy with high doses of PPI and amoxicillin avoids the problems associated with common resistance to clarithromycin, metronidazole and levofloxacin. The administration of amoxicillin 3–4 times a day in a total dose of 2–3 g is effective in preventing the decrease of concentration below therapeutic values. A meta-analysis of 15 randomised clinical trials showed that the use of PPIs and amoxicillin 4 times a day significantly improved eradication effectiveness compared to lower doses (87% vs. 73%) [112].
Quadruple therapy with bismuth is considered an effective option for second-line treatment after previous failure of quadruple therapy without bismuth. A literature review with a meta-analysis of 30 comparative studies showed that quadruple therapy with bismuth, used as a second-line treatment, had 89% effectiveness [89]. The recommended duration of second-line treatment is 14 days [22, 23, 80].
5.5. Statement 23
5.5.2. Discussion
After ineffective first- and second-line quadruple therapy with bismuth and therapy with levofloxacin, respectively, in the absence of an antibiotic sensitivity determination, the following are recommended for rescue therapy: double therapy with high doses of PPI and amoxicillin, therapy with rifabutin, quadruple therapy with bismuth and other antibiotics. The use of dual therapy with high doses of amoxicillin (4 × 750 mg) and a PPI (rabeprazole 4 × 20 mg) for 14 days as a rescue treatment had a high effectiveness (95.3%, 95% CI: 91.9–98.8%), surpassing the effects of therapy with levofloxacin or sequential treatment [113]. A meta-analysis of 12 randomised trials involving 2,249 patients confirmed that dual therapy with high-dose PPIs and amoxicillin had similar effectiveness to currently recommended regimens (83.2% vs 85.3%, RR = 1.00, 95% CI: 0.97–1.03), with a lower incidence of adverse effects (12.9% vs. 28.0%, RR = 0.53, 95% CI: 0.37–0.76) [114].
Due to the low resistance of H. pylori to rifabutin, determined to be approximately 0.13%, it can be an alternative in rescue therapy [115]. Treatment with rifabutin (2 × 150 mg or 1 × 300 mg), amoxicillin (2 × 1000 mg) and PPI (BID), as third- and fourth-line treatment, was shown to have effectiveness of 66% and 70%, respectively [116]. Based on an analysis of 3,052 patients treated with rifabutin, a successful eradication rate was confirmed in 73% of infected individuals [115]. The use of rifabutin at a daily dose of 300 mg appears to be superior over a daily dose of 150 mg. The incidence of myelotoxicity as a consequence of eradication therapy with the rifabutin regimen was less than 2%, was completely reversible and did not increase the risk of other infections [116]. In regions with an increased incidence of tuberculosis, it is important to remember that the widespread use of rifabutin may increase the risk of Mycobacterium tuberculosis resistance to this antibiotic. An alternative rescue regimen is to reuse quadruple therapy with bismuth using different antibiotics than during the previous therapy. In a Chinese study, the use of three different regimens of quadruple therapy with bismuth, based on prior antibiotic exposure, was associated with eradication effectiveness of 82.5–84% [117].
5.6. Statement 24
5.6.2. Discussion
Eradication failure is defined as a persistent positive non-serology test result for H. pylori at least 4 weeks after completion of eradication treatment according to current guidelines and after discontinuation of any medication that may affect the test sensitivity, such as proton pump inhibitors [118]. The most appropriate method for confirming H. pylori eradication is a breath test or detection of H. pylori antigens in faeces using monoclonal antibodies [22]. Determination of IgGs against H. pylori does not differentiate between active and past infection and should therefore not be used in determining the effectiveness of eradication. If an upper gastrointestinal endoscopy is planned, an alternative is to take a biopsy sample for a rapid urease test.
5.7. Statement 25
5.7.2. Discussion
In patients reporting allergy to penicillins, allergy testing is the appropriate course of action, as allergy to this antibiotic is not confirmed in most of these individuals [119]. In cases of confirmed penicillin allergy, quadruple therapy with bismuth should be used as first-line treatment [120]. Second-line treatment may benefit from triple or quadruple therapy with levofloxacin, or quadruple therapy with bismuth if not previously used [120].
5.8. Statement 26
| Only specific strains of probiotics can reduce the incidence of side effects associated with H. pylori eradication therapy. |
| Quality of evidence: moderate. Recommendation: weak. |
5.8.2. Discussion
The results of meta-analyses indicate that specific probiotic strains, through their antimicrobial and immunomodulatory effects, can support the treatment of H. pylori infection, as well as reduce the side effects of eradication treatment. The mechanism of antimicrobial action of probiotics is multifactorial and includes secreting antimicrobial substances, attenuating urease activity, increasing mucus production, inhibiting adhesion of H. pylori to gastric epithelial cells and strengthening the mucosal barrier [121]. A meta-analysis including 5,792 patients showed that probiotic supplementation improved the eradication rate by approximately 10% compared to the control group (OR = 1.94) and reduced the incidence of antibiotic-related side effects (OR = 0.56) [122]. It is important to remember that the effectiveness of probiotics depends on the species of micro-organisms used, their dosage, as well as the duration of administration. Probiotics with proven effects on reducing the incidence of side effects of H. pylori eradication treatment include Lactobacillus spp. and Saccharomyces boulardii [22]. A randomised, double-blind study showed that the administration of Lactobacillus reuteri strain DSM17648 for 2 weeks during classic triple eradication treatment resulted in a significant reduction in gastrointestinal side effects compared to placebo [123]. Probiotics with proven beneficial effects on the effectiveness of H. pylori eradication include certain strains of Lactobacillus, Saccharomyces boulardii and Bifidobacterium [22]. The results of three meta-analyses showed that the addition of S. boulardii to eradication treatment improved treatment effectiveness (RR: 1.09–1.13) [22]. One probiotic preparation combining S. boulardii and Lactobacillus reuteri DSM17648 is available on the Polish market, which, due to the unique synergistic action of these two strains, can make a significant contribution to optimising eradication treatment.
6. Prevention and public health
6.1. Statement 27
| Helicobacter pylori infection is a major etiological factor in gastric adenocarcinoma. |
| Quality of evidence: high. Recommendation: strong. |
6.1.2. Discussion
In 1994, Helicobacter pylori infection was recognised by the World Health Organisation International Agency for Research on Cancer as a Group I carcinogen, responsible for gastric cancer. Treatment of H. pylori infection has become the main method of gastric cancer prevention.
Other environmental factors for gastric cancer include Epstein-Barr virus (EBV) infection, smoking, frequent consumption of red and processed meat and high salt intake. Genetic conditions, autoimmune gastritis and status post partial gastrectomy are also factors predisposing for gastric cancer [124, 125].
6.2. Statement 28
6.2.2. Discussion
The stomach is the most common site for non-Hodgkin’s lymphomas originating from B cells (> 95%). The predominant subtype is extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT). In 90% of MALT lymphoma cases, chronic gastritis caused by H. pylori infection is found. A t(11;18)(q21;q21) translocation may be present, which is responsible for disease progression independent of antigenic stimulation, i.e. resistance to H. pylori eradication. Only about 3% of patients are found to have bone marrow involvement. In recent years, there has been a trend towards an increase in the diagnosis of gastric MALT lymphomas without concomitant H. pylori infection (6–40%). These types are characterised by a greater degree of disease progression. If no spiral bacteria are found on histology, other methods should be used to assess H. pylori infection: serology, faecal H. pylori antigen and urea breath test [126].
The primary treatment for gastric MALT lymphoma is the eradication of H. pylori. Eradication is successful in approximately 75–80% of patients, although the optimal time to achieve a treatment response can take up to 24 months. Current guidelines recommend that patients responding to antibiotic therapy should not be qualified for other forms of treatment (radiotherapy, immunotherapy).
Eradication treatment should be used even in patients with MALT gastric lymphoma and no confirmation of H. pylori infection. In patients with no lymphoma regression after antibiotic therapy and who have localised disease, radiotherapy should be used.
Helicobacter pylori infection is also likely to play a role in the pathogenesis of diffuse large B-cell lymphoma (DLBCL) and Burkitt’s lymphoma (BL) [127].
6.3. Statement 29
6.3.2. Discussion
According to ESGE (European Society of Gastrointestinal Endoscopy) recommendations, in patients with suspected gastritis in the course of H. pylori infection, it is indicate to obtain two sections from the antrum and two sections from the gastric body. The need to take sections from two sites is due to the heterogeneous distribution of the bacteria and the frequent proximal location of gastritis associated with H. pylori infection, especially in the elderly. In addition, according to the recommendations for the management of gastric precancerous conditions (MAPS II), gastric mucosal biopsies should be performed to determine the extent of atrophy and intestinal metaplasia and to establish rules for endoscopic surveillance [128].
Chronic atrophic gastritis and intestinal metaplasia (IM) are considered as pre-cancerous conditions of gastric cancer. Histological risk stratification systems (OLGA (Operative Link for Gastritis Assessment) and OLGIM (Operative Link for Gastric Intestinal Metaplasia)) should be used to assess the severity of atrophic gastritis and intestinal metaplasia. The advanced stages of atrophic gastritis are considered to be significant (moderate to severe = grades III and IV) atrophy or intestinal metaplasia that involve both the mucosa of the antrum and gastric body. Histologically confirmed intestinal metaplasia is the most reliable marker of gastric mucosal atrophy. The risk of gastric cancer is increased in patients with OLGIM grades III and IV [129, 130].
High-resolution endoscopy with chromoendoscopy shows superiority in identifying pre-cancerous lesions and early gastric cancer compared to white-light endoscopy.
To assess the severity of chronic atrophic gastritis, it is advisable to take 5 sections according to the updated Sydney protocol: 2 sections from the antrum (greater and lesser curvature; 3 cm from the pylorus), 2 sections from the body (lesser curvature; 4 cm proximal to the gastric angle and halfway through the greater curvature) and 1 section from the gastric angle – into separate containers [29, 131].
6.4. Statement 30
| In patients with chronic atrophic gastritis and H. pylori infection, we recommend eradication to reduce the risk of gastric adenocarcinoma. |
| Quality of evidence: low. Recommendation: weak. |
6.4.2. Discussion
Eradication of H. pylori promotes healing of chronic non-atrophic gastritis and may lead to regression of atrophic gastritis and reduces the risk of gastric cancer in patients with non-atrophic and atrophic gastritis [128].
In patients with confirmed intestinal metaplasia, H. pylori eradication does not appear to significantly reduce the risk of gastric cancer, but reduces inflammation and atrophy, and should therefore be considered [128].
6.5. Statement 31
6.5.2. Discussion
Patients with atrophic gastritis (mild and moderate) located only in the pre-pyloric area and around the gastric angle, without metaplasia and without risk factors (family history of gastric cancer; persistent H. pylori infection) do not require endoscopic surveillance.
Patients with atrophic gastritis and metaplasia confined to the antrum or gastric body without risk factors do not require endoscopic surveillance. However, when risk factors are present (i.e. family history of gastric cancer; persistent H. pylori infection, autoimmune gastritis) they should have a gastroscopy every 3 years.
In patients with advanced atrophic gastritis, i.e. atrophy or metaplasia in the antrum and body, OLGA/OLGIM III/IV, without a family history of gastric cancer, endoscopic surveillance (gastroscopy + sections) every 3 years is indicated due to the increased risk of gastric cancer. On the other hand, in this group of patients when close relatives have developed gastric cancer, they are subject to endoscopic surveillance every 1–2 years.
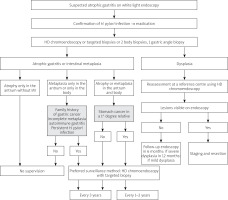
In patients with dysplasia, repeat high-resolution endoscopy with chromoendoscopy should be performed at a reference centre, and if no endoscopic lesions are found, follow-up high-resolution gastroscopy with chromoendoscopy should be performed at 12 months (low-grade dysplasia) and 6 months (high-grade dysplasia), respectively [128, 131, 132]. The management algorithm for patients with atrophic gastritis is shown in Figure 1.
6.6. Statement 32
| In patients with autoimmune atrophic gastritis, follow-up endoscopies every 3-5 years are recommended regardless of H. pylori infection status. |
| Quality of evidence: low. Recommendation: weak. |
6.6.2. Discussion
Autoimmune gastritis is a chronic, progressive inflammatory condition in which antibodies are formed against the lining cells and against Castle’s intrinsic factor. During the course of inflammation, the glandular cells of the gastric mucosa are replaced by foci of intestinal metaplasia. The inflammatory process leads to a significant reduction in hydrochloric acid secretion and the development of achlorhydria. Treatment consists of parenteral vitamin B12 supplementation and, in the case of H. pylori infection, eradication is recommended [128].
6.7. Statement 33
6.7.2. Discussion
Drugs belonging to the anticoagulants do not cause direct damage to the mucosa of the gastrointestinal tract. The mechanism of gastrointestinal bleeding is due to the disruption of the physiological haemostasis processes.
To date, few studies have been published that have assessed the association between H. pylori infection and oral anticoagulants, either vitamin K antagonists or new non-vitamin K antagonist oral anticoagulants (NOACs).
In a cohort study published in 2018, anticoagulant use was shown to increase the risk of ulcer bleeding (OR = 2.34), while no increased risk of ulcer bleeding was demonstrated in patients with H. pylori infection taking oral anticoagulants (OR = 0.95) [133].
A randomised study of a population with non-valvular atrial fibrillation using NOACs showed a 25% higher overall risk of major gastrointestinal haemorrhage complications compared to a population treated with warfarin for the same indication, with some differences between individual drugs [134]. No data available on H. pylori infection status.
As the most important risk factors for gastrointestinal bleeding in patients taking anticoagulants, most publications mark out: age over 65 years (VKAs) or over 75 years (NOACs), history of peptic ulcer disease with or without complications, concomitant use of antiplatelet drugs or NSAIDs. Confirmation of at least 1 risk factor is an indication for the use of PPI. In patients with a history of gastric or duodenal ulcers, testing for H. pylori infection is recommended before the initiation of anticoagulants [133, 135].