Introduction
Gastric cancer is the most prevalent malignant tumour of the upper digestive tract in China. Over 40% of the world’s new cases and fatalities of gastric cancer have been reported in China, with rural areas exhibiting the highest incidence rates. Early-stage gastric cancer often presents without distinct clinical symptoms, resulting in the majority of cases being diagnosed at an advanced stage. Consequently, gastric cancer ranks third among all cancer types in terms of mortality rate [1].
Currently, comprehensive therapy centred on surgery continues to be the primary approach for the treatment of gastric cancer. In recent years, laparoscopic techniques have gained widespread adoption for gastric cancer surgery. High-quality domestic and international studies have demonstrated the short-term superiority of laparoscopic radical gastrectomy over traditional open radical gastrectomy. However, studies with longer-term follow-up periods are limited [2–4]. Some scholars maintain a cautious stance towards laparoscopic techniques, particularly expressing concerns regarding the intraperitoneal dissemination of tumour cells during laparoscopic procedures. The use of high-pressure pneumoperitoneum and energy devices during laparoscopy may lead to dispersion of tumour cells throughout the peritoneal cavity, resulting in peritoneal metastasis [5, 6]. However, research in this area is relatively scarce both domestically and internationally. Therefore, in this study, we aimed to validate the safety of laparoscopic gastric cancer surgery by comparing the differences in levels of tumour markers – carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), cancer antigen 199 (CA199), and α-fetoprotein (AFP) – in the intraperitoneal drainage fluid after laparoscopic radical gastrectomy.
Aim
This study aimed to investigate variations in the levels of tumour markers in the intraperitoneal drainage fluid between laparoscopic radical gastrectomy and open radical gastrectomy for gastric cancer.
Material and methods
Clinical data
We retrospectively analysed the data of 106 patients diagnosed with gastric cancer in our Department of Gastrointestinal Surgery between July 2018 and November 2020. Pathological staging was performed as per the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Patients were categorised based on the surgical approach into a laparoscopic radical gastrectomy group (laparoscopic group) comprising 45 patients (28 men, 17 women) and an open radical gastrectomy group (open group) comprising 61 patients (43 men, 18 women). The median age in the laparoscopic group was 62.3, while in the open group it was 64.4. In the laparoscopic group, there were 17 patients with stage I, 14 with stage II, and 14 with stage III disease. In the open group, there were 11, 22, and 28 cases of stages I, II, and III, respectively.
The inclusion criteria for this study required preoperative histological confirmation of gastric cancer with clinical staging of cT2-3N0-3M0 based on abdominal computed tomography (CT) and endoscopic ultrasound examination. The exclusion criteria included distant metastasis, perforation, or severe uncontrollable systemic diseases related to gastric cancer. This study was approved by the Ethics Committee of the Affiliated Hospital of Putian University (approval number: 2020001).
Surgical quality control
Our surgical team has extensive experience in gastric cancer surgery. All operations were performed by the same surgical team to ensure consistency in both the procedure and postoperative care.
Surgical procedure
All gastric cancer operations adhered to the standard guidelines for radical gastric resection as per the 6th edition of the Japanese Gastric Cancer Treatment Guidelines and included complete D2 lymph node dissection [7]. Modified Roux-en-Y (with P-loop) reconstruction was performed for gastrointestinal continuity in total gastrectomy (TG), whereas Billroth II anastomosis was employed in distal gastrectomy (DG) reconstruction. The incision length for the laparoscopic group was approximately 8 cm, and for the open group it was approximately 18 cm; both were performed through a midline upper abdominal incision.
Detection methods
Upon admission, all patients underwent peripheral venous blood tests for CEA, CA125, CA199, and AFP levels. Additionally, 5 ml of the peritoneal drainage fluid was naturally collected via gravity using a peritoneal drainage tube on postoperative days (PODs) 1, 2, 3, and 5 for laboratory testing. After centrifugation at 3000 rpm for 10 min, the supernatant was analysed using the Roche E601 instrument and the corresponding reagent kits through a chemiluminescent immunoassay. The normal ranges of tumour biomarkers in serum were as follows: CEA, 0–5 ng/ml; CA125, 0–35 U/ml; CA199, 0–27 U/ml; AFP, 0–7 ng/ml.
Statistical analysis
Data analysis was performed using SPSS version 25.0. Continuous data were presented as mean ± standard deviation (SDs). The independent-samples t-test or one-way analysis of variance was applied to normally distributed data, whereas non-normally distributed data were subjected to nonparametric tests. Skewed data were analysed using the nonparametric Mann-Whitney U test. Survival analysis was performed using the Kaplan-Meier method using GraphPad 8.0 software, and the log-rank test was employed to assess differences, with significance set at p < 0.05.
Results
In total, 106 patients were included in this study. The baseline characteristics and pathological stages of the patients are presented in Table I. The median age in the laparoscopic and open groups were 62.31 and 64.41 years, respectively. Comparison between the laparoscopic and open groups revealed no statistically significant differences in clinical data. There were no discernible differences in sex distribution, age, pathological stage, or type of gastrectomy between the two groups (p > 0.05) (Table I).
Table I
Comparison of clinical data between the laparoscopic and open surgery groups
Preoperative peripheral blood levels of CEA, CA125, CA199, and AFP from 50 patients with non-tumour conditions, such as gastrointestinal polyps and acute appendicitis, during the same period as the normal group, were selected. The differences in biomarker levels among the three groups were then compared. Comparison between the laparoscopic, open, and control groups revealed no statistically significant differences in the preoperative peripheral blood levels of CEA, CA125, CA199, and AFP (p > 0.05) (Table II).
Table II
Comparison of preoperative tumour markers in peripheral blood among the laparoscopic surgery, open surgery and control groups
The laparoscopic and open groups exhibited no significant differences in postoperative CEA levels on POD 1 (p > 0.05), POD 2 (p > 0.05), POD 3 (p > 0.05), and POD 5 (p > 0.05) (Table III). Regarding CA125 levels, although the difference was not significant on POD 1 (p > 0.05), the values were close to statistical significance. On POD 2, the difference in CA125 levels between the laparoscopy and open groups (338.84 ±203.34 U/ml vs. 248.11 ±165.90 U/ml) was statistically significant (p < 0.05). However, on POD 3 and POD 5, the difference was not statistically significant (p > 0.05) (Figure 1). Regarding the levels of CA199 in postoperative drainage fluid, there were no significant differences on POD 1 (p > 0.05), POD 2 (p > 0.05), POD 3 (p > 0.05), and POD 5 (p > 0.05) between the laparoscopic and open groups. No significant differences were observed in postoperative abdominal drainage fluid AFP levels between the two groups on POD 1 (p > 0.05), POD 2 (p > 0.05), POD 3 (p > 0.05), and POD5 (p > 0.05) (Table III).
Table III
Comparison of CEA, CA199, and AFP levels in drainage fluid after surgery between the laparoscopic and open groups
Figure 1
Comparison of CA125 levels in drainage fluid after surgery between the laparoscopic and open groups across various pathological stages. A – Histogram of CA125 levels in the postoperative drainage fluid of gastric cancer patients undergoing laparoscopic surgery compared to the open surgery group on PODs 1, 2, 3 and 5 (**p < 0.05, N = 45–61). B – Comparison of CA125 levels in drainage fluid after surgery between groups for stage I gastric cancer patients on PODs 1, 2, 3 and 5 (**p < 0.05, N = 11–17). C – Comparison of CA125 levels in drainage fluid after surgery between groups for stage II gastric cancer patients on PODs 1, 2, 3 and 5 (**p < 0.05, N = 14–22). D – Histogram showing CA125 levels in drainage fluid after surgery between groups in stage III gastric cancer patients on PODs 1, 2, 3 and 5 (N = 14–28). PODs – postoperative days
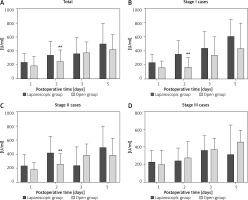
We conducted a comparative analysis of stage I cases between the laparoscopic and open groups. On POD 2, CA125 levels in the laparoscopic group were notably higher than those in the open group (p < 0.05). However, no statistically significant differences were found between the two groups on POD 1, POD 3, and POD 5 (Figure 1). Furthermore, the postoperative levels of CEA, CA199, and AFP were not significantly different between the two groups on PODs 1,2, 3, and 5 (Table IV).
Table IV
Comparison of CEA, CA199, and AFP levels in drainage fluid after surgery between the stage I cases of the laparoscopic and open groups
Subgroup analysis of stage II cases in both groups revealed that on POD 2, CA125 levels in the laparoscopic group were significantly higher than those in the open group (p < 0.05). However, no statistically significant differences were found between the two groups on POD 1, POD 3, and POD 5 (p > 0.05) (Figure 1). Similarly, levels of CEA, CA199, and AFP showed no significant differences between the two groups on PODs 1, 2, 3, and 5 (p > 0.05) (Table V).
Table V
Comparison of CEA, CA199, and AFP levels in drainage fluid after surgery between the stage II cases of the laparoscopic and open groups
In the subgroup analysis of stage III cases, no statistically significant differences were observed in the levels of CEA, CA125, CA199, and AFP between the laparoscopic and open groups on PODs 1, 2, 3, and 5 (p > 0.05) (Figure 1, Table VI).
Table VI
Comparison of CEA, CA199, and AFP levels in drainage fluid after surgery between the stage III cases of the laparoscopic and open groups
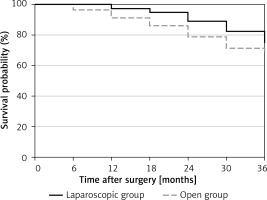
After excluding 5 cases lost to follow-up, we conducted a follow-up analysis of the 3-year survival rates of both groups. The difference in 3-year survival rates between the laparoscopic and open groups (81.4% and 70.7%) was not statistically significant (p = 0.3794) (Figure 2).
Discussion
Gastric cancer, the most prevalent malignant tumour of the upper digestive tract in China, is primarily treated with laparoscopic radical gastrectomy. In 1994, Kitano successfully performed the first laparoscopic distal gastrectomy [8]. Subsequently, laparoscopic gastric cancer surgery has gained global popularity, with several leading gastrointestinal cancer centres in various countries conducting numerous clinical trials and studies on this approach [2–4, 9]. The CLASS-01 trial initiated by Li et al. revealed no significant difference in the 3-year disease-free survival rate between patients with locally advanced gastric cancer who underwent laparoscopic gastrectomy and those who underwent open distal gastrectomy in China. This suggests that laparoscopic gastric cancer surgery may benefit patients in the perioperative period, with no significant differences observed during the long-term follow-up compared to open surgery [4].
Peritoneal metastasis is a key prognostic factor for gastric cancer. Approximately 20–50% of patients with advanced gastric cancer experience peritoneal metastasis after surgery. Once peritoneal metastasis occurs, the survival time sharply declines, and the median survival period generally does not exceed 1 year [10]. The mechanism underlying the peritoneal metastasis of gastric cancer remains elusive. Currently, the “seed and soil” theory is widely accepted, which suggests that the occurrence of peritoneal metastasis depends on the cancer cells and peritoneal microenvironment [11]. Experts such as Gutt and Wittich believe that laparoscopic surgery entails a prolonged operation time, and the maintenance of a CO2 pneumoperitoneum along with the vaporisation phenomenon while operating using an ultrasonic scalpel may promote the proliferation of gastric cancer cells in the abdominal cavity and enhance their adhesion ability, potentially resulting in peritoneal metastasis [5, 6]. West and others believe that CO2 pneumoperitoneum and surgical procedures disrupt the balance of the intra-abdominal microenvironment, which may inhibit the secretion of cytokines such as tumour necrosis factor (TNF)-α from intra-abdominal macrophages, leading to a reduction in local immune function in the abdomen and further facilitating the invasion and metastasis of gastrointestinal tumour cells [12, 13]. Moreover, the “chimney effect” caused by laparoscopic surgery may also lead to incisional implantation metastasis, increasing the probability of postoperative peritoneal metastasis [14].
Pathological evidence of intraperitoneal shedding of cells or biopsy tissues remains the gold standard for diagnosing peritoneal metastasis. However, detecting intraperitoneal shedding cells, particularly during and after surgery, is complicated and exhibits low positivity rates and specificity. Many domestic and international researchers have attempted to detect tumour markers in peritoneal drainage fluid to predict the occurrence of peritoneal metastasis. CEA levels are commonly used to predict peritoneal metastasis following gastric cancer surgery. Previous studies indicate a close association between CEA levels in the peritoneal washes and peritoneal recurrence, suggesting that CEA levels can be used as a prognostic tool, with elevated levels in the peritoneal washes during gastric cancer surgery indicating a higher likelihood of postoperative peritoneal metastasis [15–17]. Several studies have demonstrated a significant elevation in CEA levels within the postoperative peritoneal drainage fluid of patients with gastrointestinal tumours. This surge in CEA levels has been closely correlated with tumour invasion depth, lymph node metastasis, and clinical staging, thereby serving as a potential prognostic indicator [18, 19]. Wei et al. inferred that laparoscopic radical gastrectomy does not increase the shedding of gastric cancer cells by comparing CEA levels in the peritoneal lavage fluid [20]. In our study, the CEA levels in the preoperative and postoperative peritoneal drainage fluid between the laparoscopic and open groups showed no significant differences on PODs 1, 2, 3, and 5 (p > 0.05). These findings are consistent with domestic and international research results and support the notion that laparoscopic radical gastrectomy does not increase the risk of postoperative peritoneal metastasis.
CA125 is a typical tumour marker for ovarian cancer but is also considered a marker of peritoneal metastasis in gastric cancer. Previous studies have shown a close relationship between CA125 and peritoneal metastasis in patients with gastric cancer [21, 22]. Our study found no significant difference between the laparoscopic and open groups on PODs 1, 3, and 5 (p > 0.05). However, there was a significant increase in the laparoscopic group compared with the open group on POD 2 (p < 0.05). In the subgroup analysis of stage I and II cases, a notable elevation in CA125 levels was observed in the laparoscopic group on POD 2. CA125 is secreted by epithelial cells in the body cavity. It has high sensitivity but low specificity. It is closely associated with abdominal inflammation and ascites. There has been limited research on the levels of CA125 in peritoneal drainage fluid. However, one study has found that postoperative CA125 levels in peripheral blood increase daily, with a peak occurring on the 10th postoperative day (range: 7–30 days), the median normalization day of CA125 levels in preoperative normal and postoperatively elevated patients was 57 days (range: 28–115 days) [23]. In our study, we observed a similar trend, with CA125 levels in the peritoneal drainage fluid increasing daily in the first 5 days postoperatively. In the first 2 days after surgery, the elevated CA125 levels in the open group may be due to a large amount of peritoneal lavage during the open procedure, while the laparoscopic group only underwent localised lavage. Peritoneal lavage can reduce the release of inflammatory mediators from tissues during surgery, clear residual necrotic tissue, blood clots, and contaminated or infected fluids, and shed tumour cells. This reduces the risk of infection at the surgical site and minimises peritoneal irritation and wound exudation, thereby reducing abdominal inflammation and lowering CA125 levels. By POD 3 and POD 5, the peritoneal microenvironment tended to stabilise; therefore, there was no significant difference between the two groups. Therefore, we believe that the isolated elevation of CA125 in the drainage fluid on the second day after laparoscopy does not confirm its association with tumour dissemination. Further research is required to determine the significance of this elevation.
Additionally, we compared the changes in CA199 and AFP levels in the drainage fluid between the two groups. CA199 is an important tumour marker for pancreatic cancer; however, a previous study found that CA199 was also significantly increased in patients with peritoneal metastasis from gastric cancer [24]. The results of this study indicated that there were no statistically significant differences in the levels of CA199 in the peritoneal drainage fluid between the laparoscopic group and open group on PODs 1, 2, 3, and 5 (p > 0.05). AFP is a crucial tumour marker for liver cancer; however, in some patients with gastric cancer, AFP levels markedly increase. Studies have suggested a close association between elevated AFP levels and the progression of gastric cancer and occurrence of peritoneal metastasis [25, 26]. In this study, we found no significant differences in AFP levels between the laparoscopic group and open group on PODs 1, 2, 3, and 5, and no statistical significance in the comparison between the two groups (p > 0.05). This further confirms that laparoscopic radical gastrectomy does not increase the risk of postoperative peritoneal metastasis.
Our research revealed that there were no statistically significant differences in the levels of CEA, CA125, CA199, and AFP in the peritoneal drainage fluid between the laparoscopic and open groups of patients with gastric cancer patients on PODs 1, 2, 3, and 5. Furthermore, comparison of the 3-year postoperative survival rates between the two groups (81.4% vs. 70.7%) did not show any statistically significant difference. Consequently, laparoscopy is safe for radical gastrectomy and does not increase the risk of postoperative peritoneal metastasis. Peng et al. reported that CO2 pneumoperitoneum itself has no substantial impact on the growth, proliferation, and migration of gastrointestinal tumour cells. Furthermore, it does not promote the spread of tumour cells within the peritoneal cavity. Heating CO2 is suggested to inhibit intra-abdominal tumour dissemination [27, 28]. Additionally, Hao et al. demonstrated that laparoscopic surgery minimises physical trauma to patients. The likelihood of free cancer cells appearing during the operation is minimal, with a low risk of micro-metastasis postoperatively [20, 29].
We believe that laparoscopic surgery provides a clearer surgical field of view, particularly with the utilisation of new technologies, such as 4K laparoscopy and indocyanine green near-infrared imaging. These advancements have enabled more precise vascular and lymphatic dissections, leading to sharp anatomical tumour dissection and thorough lymph node clearance. This reduces the risk of intraoperative bleeding and lymphatic leakage, thereby avoiding potential tumour cell dissemination [30, 31]. Moreover, laparoscopic surgery allows for a more precise exposure, avoiding direct manipulation of the tumour itself. This reduces prolonged traction, compression, and flipping of the tumour, as typically seen in open surgery, consequently minimising the direct stimulation of the tumour mass. Specifically, the application of laparoscopic gastric cancer mesentery dissection techniques permits maximal removal of the tumour and mesentery, thus minimising “cancer spillage” and adhering to the principle of tumour surgery with a “no-touch” technique. This approach reduces the risk of tumour cell shedding and implantation [32].
However, owing to the limited number of cases included in this study, there are inherent limitations. Future research supported by more data and molecular mechanism studies is anticipated to further validate the impact of laparoscopic surgery on peritoneal metastasis.
Conclusions
In our study, there were no significant differences in CEA, CA125, CA199, and AFP levels in the intraperitoneal drainage fluid between laparoscopic radical gastrectomy and open radical gastrectomy for gastric cancer on POD 1, POD 2, POD 3, and POD 5, suggesting that laparoscopic radical gastrectomy does not increase the risk of intraperitoneal metastasis.