Introduction
Surgical aortic valve replacement (SAVR) requires cardio-pulmonary bypass (CPB) and cardiac arrest [1]. Suboptimal intraoperative myocardial protection may have a negative impact on clinical outcomes, particularly in patients with aortic valve stenosis (AVS)-related hypertrophic left ventricle [2]. So far, numerous methods have been suggested to prevent CPB-associated cardiomyocytes injury [3]. One of the most crucial is cardioplegic arrest [3, 4]. Up until now, many types of them have been introduced, varying in content, temperature, method of application, and many other aspects [4–6]. Among them, St Thomas Hospital II (STH II) crystalloid cardioplegic formula was a standard in cardiac surgery for many years [7]. It is worth noting that in recent years, it has been supplanted by others. However, it is still considered as a reference in many studies evaluating novel formulas or assessing the particular aspects of intraoperative injury in experimental animal models [7, 8].
Before SAVR, surgeons together with anesthetists must determine the optimal dose and volume of cardioplegia. Aforementioned calculations are usually based on the body mass [9]. However, one must be aware that aortic valve stenosis, particularly when it has been developing for many years prior to the operation, is usually associated with extensive left ventricular (LV) myocardial hypertrophy [10]. Therefore, the aforementioned strategy of cardioplegia dose/volume determination, i.e. exclusively on the basis of the patient’s body mass, may be linked to substantial bias.
Aim
The aim of our study was to assess whether the classical protocol of STH II application is safe in all patients undergoing SAVR for isolated severe aortic stenosis (AS) and whether cardioplegia dose has any association with postoperative myocardial injury and long-term survival.
Material and methods
Patients
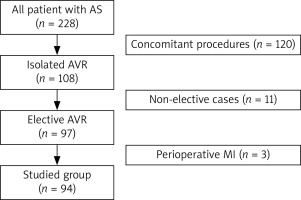
This study involved 94 consecutive elective patients (48 males and 46 females) with a mean age of 65.4 ±7.8 years who underwent isolated SAVR in CPB for significant aortic valve stenosis in 2016 at a single cardiac surgical center. Therefore AS individuals with concomitant cardiac diseases requiring surgical interventions and those prioritizing urgent or emergent treatment were excluded. Additionally, patients who developed myocardial infarction throughout the preoperative period were not included in our analysis. Details of the selection process for this study are presented in Figure 1. The demographic data of the examined population are outlined in Table I.
Table I
Demographic and selected clinical data
| Parameter* | All (n = 94) | Subgroup A (n = 47) | Subgroup B (n = 47) | P-value# |
|---|---|---|---|---|
| Age [years] | 65.4 ±7.8 | 65.7 ±7.9 | 65.1 ±7.8 | 0.975 |
| Gender M/F, n (%) | 48 (51.1) | 21 (44.7)/26 (55.3) | 27 (57.4)/20 (42.6) | 0.216 |
| Weight [kg] | 83.2 ±16.9 | 82.7 ±19.2 | 83.7 ±14.4 | 0.758 |
| Height [m] | 1.66 ±0.09 | 1.64 ±0.09 | 1.68 ±0.09 | 0.161 |
| BMI [kg/m2] | 30.2 ±5.2 | 30.7 ±5.9 | 29.8 ±4.4 | 0.422 |
| Obesity&, n (%) | 58 (53.2) | 30 (63.8) | 20 (42.6) | 0.039 |
| BSA | 1.90 ±0.22 | 1.88 ±0.24 | 1.93 ±0.19 | 0.314 |
| Arterial hypertension, n (%) | 64 (68.1) | 33 (70.2) | 31 (66.0) | 0.658 |
| Hyperlipidemia, n (%) | 20 (21.3) | 12 (25.5) | 8 (17.0) | 0.313 |
| Diabetes mellitus, n (%) | 28 (29.8) | 18 (38.3) | 10 (21.3) | 0.071 |
| CAD, n (%) | 28 (29.8) | 13 (27.7) | 15 (31.9) | 0.652 |
| ACS in history, n (%) | 10 (10.6) | 6 (12.8) | 4 (8.5) | 0.738 |
| AF in history, n (%) | 28 (29.8) | 16 (34.0) | 12 (25.5) | 0.367 |
| PAD, n (%) | 2 (2.1) | 2 (4.3) | 0 | 0.475 |
| COPD, n (%) | 18 (19.1) | 6 (12.8) | 12 (25.5) | 0.116 |
Figure 1
Patient selection process. Non-elective patients as well as those requiring additional procedures were excluded from the current study
AS – aortic stenosis, AVR – aortic valve replacement, MI – myocardial infarction.
Due to the fact that this study was exclusively a retrospective analysis of the medical charts, we did not apply to the Local Bioethical Committee for approval. Of note, all patients expressed informed consent for surgical treatment of aortic stenosis, and the principles outlined in the Declaration of Helsinki were respected.
Preoperative clinical and echocardiographic assessment
Before surgery, all study participants underwent clinical evaluation (history analysis and physical examination), laboratory, and transthoracic echocardiographic (M + 2D + Doppler) studies (Tables I, II). In the latter one, the basic morphological and functional parameters were recorded. In addition, for this study, left ventricular mass (LVM) was calculated using the Devereux formula with later modifications (LVM = 0.8 × (1.05 (IVSd + LVPWd + LVEDd)3) – LVEDd 3 + 0.6 g) [11, 12]. The LVM index (LVMI) was then calculated using the body surface area (BSA) (LVMI = LVM/BSA (g/m2)). If LVMI exceeded 95 g/m2 in women and 115 g/m2 in men, LVH was defined as mild (96–108 g/m2 for women; 116–131 g/m2 for men), moderate (109–121 g/m2 for women; 132–148 g/m2 for men), and severe (122 g/m2 for women; 149 g/m2 for men) [12, 13].
Table II
Preoperative findings in transthoracic echocardiography
| Parameter* | All (n = 94) | Subgroup A (n = 47) | Subgroup B (n = 47) | P-value# |
|---|---|---|---|---|
| LVEDd [cm] | 4.40 ±0.82 | 4.06 ±0.72 | 4.74 ±0.79 | < 0.001 |
| LVESd [cm] | 2.83 ±1.36 | 2.57 ±1.11 | 3.07 ±1.54 | 0.032 |
| IVSd [cm] | 1.65 ±0.30 | 1.58 ±0.21 | 1.72 ±0.36 | 0.023 |
| LVPWd [cm] | 1.62 ±0.38 | 1.50 ±0.36 | 1.74 ±0.33 | < 0.001 |
| LVM [g] | 312 ±106 | 247 ±77 | 377 ±112 | < 0.001 |
| LVMI [g/m2] | 163 ±56 | 130 ±30 | 196 ±55 | < 0.001 |
| LVH&: | 84 (89.4) | 37 (78.7) | 47 (100.0) | 0.003 |
| Mild | 2 (2.4)$ | 2 (5.4) | 0 | 0.372 |
| Moderate | 16 (19.0) | 14 (37.8) | 2 (4.3) | 0.003 |
| Severe | 66 (78.6) | 21 (56.8) | 45 (95.7) | < 0.001 |
| RVd [cm] | 2.98 ±0.87 | 2.89 ±0.55 | 3.05 ±1.09 | 0.132 |
| LA [cm] | 3.90 ±0.80 | 3.84 ±0.57 | 3.98 ±1.00 | 0.247 |
| LVEF [%] | 61.2 ±11.1 | 69.3 ±12.3 | 57.2 ±10.4 | < 0.001 |
| LVEF < 40%, n (%) | 5 (5.3) | 1 (2.1) | 4 (8.5) | 0.358 |
| Regional contractility abnormalitie, n (%) | 7 (7.4) | 2 (4.3) | 5 (10.6) | 0.432 |
| PPG [mm Hg] | 97.8 ±24.9 | 104.0 ±27.2 | 92.7 ±23.3 | 0.271 |
| MPG [mm Hg] | 53.1 ±16.0 | 55.0 ±16.1 | 49.8 ±15.7 | 0.437 |
* Continuous data are presented as mean with standard deviation (all met the criteria of a normal distribution in the Shapiro-Wilk W test);
# it applies to comparison of subgroup A vs. B, bolded if differences are statistically significant;
& defined according to the American Society of Echocardiography (see ‘Patients and Methods’ section);
IVSd – interventricular septum thickness in diastole, LA – left atrial dimension, LVEDd – left ventricular end-diastolic dimension, LVEF – left ventricular ejection fraction, LVESd – left ventricular end-systolic dimension, LVH – left ventricle hypertrophy, LVM – left ventricular mass, LVMI – left ventricular mass index, MPG – mean systolic pressure gradient, PPG – peak systolic pressure gradient, RVd – right ventricular dimension.
Surgery details
All operations were performed through the median sternotomy in CPB with aortic and bicaval cannulation and in moderate hypothermia (29–30°C). As intraoperative myocardial protection, crystalloid cardioplegia according to the STH II formula was administered directly to the coronary ostia with an initial dose of 10 ml/kg body mass, repeated every 20 min of myocardial ischemia (e.g., when the ascending aorta was cross clamped). Severely stenotic valves were removed completely and replaced with either pericardial biological (Carpentier-Edwards PERIMOUNT Magna, Edwards Lifesciences, Irvine, CA, USA) or mechanical (OnX prosthetic heart valve; On-X Life Technologies, Inc., Austin, Texas, USA) valves. After the aortic clamp was released, special attention was paid to the completion of the valid reperfusion phase of CPB. Eventually, the chest was closed in a standard manner, and patients with stable hemodynamic status were transferred to the postoperative intensive care unit (PICU).
Overall doses (in ml) of cardioplegia given intracoronarily were collected from the medical charts of CPB, and then they were referred to LVM (indexed cardioplegia volume (ml/g of LV myocardium)) calculated on the basis of findings in the preoperative echocardiographic examination. Eventually, the studied patient group was divided into two subgroups: subgroup A with a dose of indexed cardioplegia higher than 4.49 ml/g of LV myocardium (median; Q2), and subgroup B with lower doses (below the median).
Postoperative course evaluation
At the PICU, postoperative care involved mechanical ventilation within the first few hours after surgery, and strict monitoring of all vital systems, with particular attention to stability of systemic circulation. At the same time, blood samples were withdrawn from peripheral arteries (usually one of the radial arteries) on a regular basis. For study purposes, cardiac troponin I (cTnI) concentrations at 1, 24, 48 and 72 hours after surgery were extracted from the medical charts. Their maximal values were also used in the further analyses (cTnI max). Individuals who were hemodynamically stable were transferred to the surgical ward before being discharged to rehabilitation centers on the 7th to 10th postoperative day.
Early (up to 30 days after surgery), middle- (up to one year) and long-term mortality rates were estimated on the basis of official information of the Central Statistical Office.
Data management and statistical analysis
Continuous data were checked for normality using the Shapiro-Wilk W test. Then those that satisfied criteria for a normal distribution were presented as the mean with standard deviation (SD) and then compared between subgroups (A vs. B) by means of an unpaired Student’s T test. Otherwise, they were expressed as the median (Q2) with an interquartile range (IQR: 1st to 3rd quartile) and then analyzed statistically by the non-parametric Mann-Whitney U test. Categorical variables were presented as numbers (n) with percentages (%) and then compared by means of the χ2 test with Yates correction if applicable. The correlations between the total dose of cardioplegia administered intraoperatively and peak postoperative cTnI concentrations were tested using Pearson’s correlation coefficient. In the latter, a strong relationship was defined if the value of r was above 0.5, moderate if between 0.3 and 0.5, and weak or none if below 0.3. Early and one-year mortality rates were presented as numbers and percentages, while long-term survival probabilities were stratified using the Kaplan-Meier curves. The log rank test was applied to compare the curves for subgroup A and B subjects. The p-values in all the aforementioned tests were considered to be statistically significant. The analyses were performed using Statistica 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA).
Results
Intraoperative data
Two elderly patients (75 and 77 years old) were disqualified from SAVR after intraoperative inspection that revealed a severely atherosclerotic ascending aorta. Both underwent successful transcatheter aortic valve implantation (TAVI) a few months later.
During operations in a mean CPB time of 81.5 ±10.8 min (80.7 ±10.0 min in subgroup A and 82.2 ±11.4 min in subgroup B, p = 0.492) and a cross-clamping time of 62.3 ±10.8 min (61.1 ±8.4 min in subgroup A and 63.4 ±12.7 min in subgroup B, p = 0.299), two to three doses of STH II solution were infused to the coronary circulation. Because the patients in both subgroups were comparable in terms of body height, weight and BSA, the total volume of cardioplegia administered intraoperatively (1403 ±318 ml vs. 1360 ±236 ml, in subgroups A and B, respectively) was also comparable (p = 0.454). However, if we estimated the total dose of STH II solution (ml) per gram of the LV myocardium, a statistically significant difference was observed between subgroups. A much higher volume of cardioplegia was administered in subgroup A (6.0 ±1.4 ml/g) than in subgroup B subjects (3.8 ±0.7 ml/g) (p < 0.001).
Postoperative myocardial injury
A marked increase in cTnI was noted in all patients undergoing SAVR. Its peak value was usually observed 24 hours after procedures (median (IQR) for whole group: 8.415 (6.071, 15.471 μg/l)) and then started to decline. Of note, the concentration of cTnI remained increased even up to 48 hours later (i.e., 3 days after surgery). However, its release from the myocardium was much more pronounced in subgroup B than A (Table III). Moreover, its maximal concentration throughout the first 72 postoperative hours was also markedly higher in subgroup B (14.918 (10.710; 26.195)) than in A (9.876 (7.070; 12.454)) (p = 0.005).
Table III
Perioperative concentrations of cTnI in the studied subgroups
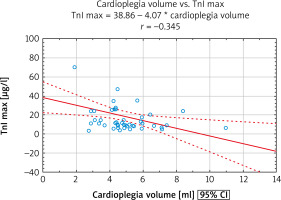
For the whole group of SAVR subjects, a moderate negative correlation between volume of infused cardioplegic solution indexed for LVM (LVMI) and cTnI max in the early postoperative period was found (Figure 2).
Figure 2
Relationship between cardioplegia volume and intraoperative myocardial injury. A significant negative correlation between cardioplegia volume per gram of myocardium and peak concentration of cTnI within the first 72 hours after SAVR was found. Of note, it applied to the whole group of patients
cTnI – cardiac troponin I, cTnI max – maximal concentration of cardiac troponin I, SAVR – surgical aortic valve replacement.
Intra- and post-SAVR survival
All SAVR patients survived the initial postoperative period. Four deaths (2 cases in each subgroup) occurred within the first year of follow-up. Therefore, one-year mortality was 4.3% in both subgroups. Contrary to middle-term mortality, in later follow-up, four other fatal cases involved only subgroup B. As a result, the Kaplan-Meier curve analysis revealed that the probability of survival in group B was significantly lower than in group A. In detail, the one- and five-year survival probabilities in group A were 95.8% and 95.8%, respectively, compared to 95.7% and 82.6% in group B (p = 0.044) (Figure 3).
Discussion
The principal finding of this preliminary study is that intraoperative myocardial injury estimated by means of cTnI release depends predominantly on the dose of infused cardioplegic solution indexed for LV mass. However, it must be stressed that such estimation of the volume of cardioprotective infusion is not routine in daily cardiac surgical practice [14]. We found that calculations based solely on the patient’s body mass (a common strategy) were suboptimal, particularly in AS patients with excessive LVH. Of note, the vast majority of our patients, even in subgroup B, presented with LVH due to advanced AS. Similar findings were reported by Condello et al. in an elegant study comparing crystalloid cardioplegia arrest indexed to LVMI versus body weight or surface area [15]. They had a lower risk of developing post-AVR low cardiac output syndrome. However, their study involved only the early postoperative period.
We are not the first to claim how important optimal myocardial protection is during AVR in patients with hypertrophic LV myocardium. Even many years ago, some surgeons suggested using a higher volume of cardioplegia for surgical AVR in patients with hypertrophic LV [16]. Their recommendations were supported exclusively by the early laboratory findings: creatine phosphokinase-MB (CK-MB) or cTn concentrations or in-hospital course. Later, when comparing warm blood to cold crystalloid cardioplegia, Nardi et al. found similar clinical outcomes in general, with the exception of significant LV hypertrophic patients. Cold rather than warm cardioplegic intraoperative arrest may provide better protection in the latter cases [17]. In the last few years, more sufficient strategies regarding cardioplegia have been described, including the administration of histidine-tryptophan-ketoglutarate (HTK) or del Nido solutions [18–20]. Currently, at our department, HTK solution is being used, but as in our study, the earlier period was taken into consideration to investigate the late survival; hence the cardioplegic solution administered to the patients included in the study was STH II cardioplegia.
It was also found in this study that an underestimated cardioplegic dose had a negative impact on long-term survival. Patients with higher doses of cardioplegia (i.e., subgroup A) presented not only with decreased release of myocardial injury markers but also with a more favorable probability of long-term survival following surgical AVR. The latter finding was not reported previously. However, when discussing the issue of group survival, we must comment on at least a few issues. First of all, our group’s 30-day mortality was lower than previously reported [21–23]. Risk factors of a negative impact on early mortality have already been well defined [22, 24]. It must be noted that our observations involved only elective cases with isolated valve disease. Moreover, in 2016 TAVI procedures were routinely performed at our center, particularly in high risk patients, including the elderly population. Age 80 and older has previously been identified as a risk factor for both in-hospital and post-discharge morbidity and mortality [25]. The perfect 30-day outcomes of patients recruited to this study might have resulted from the fact that more than 90% of them were considered low-risk for mortality and underwent relatively simple surgery.
In general, the 1- and 5-year probabilities of survival in our group were comparable to the previous reports [22–26]. We are not surprised that post-discharge follow-up outcomes were worse in subjects with lower doses of cardioprotective infusions indexed for LV mass. We cannot rule out the possibility that more pronounced LV hypertrophy, rather than the dose, may influence the likelihood of survival after SAVR. Hypertrophy, but not the severity of aortic valve stenosis, was previously shown to be a significant risk factor for sudden cardiac arrest [27]. Positive remodeling is not seen in all patients, even after an uneventful surgical AVR with no patient-prosthesis mismatch following surgery. Of note, residual HT in such individuals was shown to be associated with decreased long-term survival [28]. Additionally, comparison of selected preoperative clinical parameters between groups A and B revealed significant differences. Some of them, such as LVEF or prevalence of obesity, may have a potential impact on postoperative survival. Lower LVEF is a well-known predictor of unfavorable outcomes, but it usually applies to patients with significantly reduced systolic LV performance (i.e. LVEF below 40%) [29]. Therefore, it is rather unlikely that the markedly lower mean value of this parameter had any influence on postoperative prognosis as the prevalence of low LVEF cases was comparable between groups. We are also have in mind the “obesity paradox” indicating that overweight and obese class I or II individuals undergoing cardiac surgical procedures (more obese in class I in group A than B) may have better survival than subjects with a normal BMI value [30].
The authors of this study are aware of some limitations. First of all, the number of patients included in the study is limited to just one year of surgical activity at a middle-volume cardiac surgical center. However, consecutive patients who fulfilled the entry criteria (elective isolated AS) were involved in this study. This assessment was a retrospective one, but real-life data were analyzed. The LVM was calculated on the basis of an echocardiographic study. Although the applied method has been approved for many years by the European Society of Echocardiography, it poses known limitations. The suggested method is reliable only in patients with well-preserved ejection fraction of the left ventricle and its normal geometry. Only one case in our group had LVEF less than 40% and/or regional LV myocardial contractility disturbances prior to surgery.
Conclusions
This preliminary study suggested that the calculation of cardioplegia volume should not be based solely on body mass. One should also consider taking into account the echocardiographically calculated left ventricular mass. It is likely that higher extents of myocardial injury stratified on the basis of maximum concentration of troponin I may negatively affect the late survival of subjects undergoing isolated aortic valve replacement.







