Introduction
Bone wax use is aimed at diminishing oozing from bone marrow after median sternotomy, but its protective effect on intra- and postoperative bleeding still remains controversial [1, 2]. All previous analyses have been performed only in cardiac surgery using a cardiopulmonary bypass. No studies have evaluated the utilization of bone wax in off-pump settings yet. Moreover, the long-term effect on sternal wound healing by liberal use of bone wax could not be omited [3].
Aim
The aim of this study was to evaluate the benefit of bone wax on intraoperative blood loss in high-risk off-pump coronary artery bypass grafting (OPCAB) patients and its potential impact on cell saver use. The number of transfusions and the total incidence of sternal wound infection (SWI) were observed.
Material and methods
This was a prospective randomized study of patients undergoing off-pump coronary surgical revascularization at our institution between the years 2013 and 2019. Inclusion criteria were: diabetes; two-vessel disease to be revascularized by left internal thoracic artery (LITA) and the venous graft; written informed consent. Exclusion critera were: re-do surgery, known allergy to gentamicin, continuing anticoagulation or antiplatelet therapy except aspirin. Patients with incomplete revascularisation or extracorporeal circulation (ECC) use were additionally excluded from the analysis.
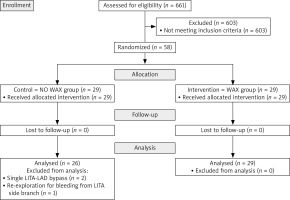
All included patients were randomly assigned to a wax group (W) or no wax group (NW) using an even-odd method by the principal investigator of the study. They were operated on by two experienced consultant cardiac surgeons engaged in beating heart surgery. Intraoperative blood loss in a waste container was measured at the end of the operation and further analyzed. All the perioperative outcomes until discharge were recorded. Every patient was contacted by phone 3 months postoperatively and incidence of SWI was recorded. The study flow diagram is presented in Figure 1. The study was approved by the local ethics committee (Ethics Committee at the University Hospital Hradec Králové, Reference Number: 201 402 514 P). Informed consent was obtained from all patients. The study dataset will be shared upon reasonable request to the corresponding author.
Primary outcomes were the intraoperative blood loss and the incidence of SWI in the first 3 months postoperatively. Secondary outcomes included other standard peri- and post-operative measures. Multivariate analysis of the risk of high intraoperative blood loss was additionaly performed.
Anesthetic and surgical technique
The anesthetic technique consisted of propofol infusion at 1 mg/kg/h combined with sufentanil infusion at 0.5 μg/kg/h. Neuromuscular blockade was achieved by 0.04 mg/kg/h cisatracurium. Patients were normocapnic ventilated using isoflurane (Forane, Abbot Laboratories, GB; 0.7–0.8 %). Intravenous Unasyn (sulbactam/ampicillin) and later on Azepo (cefazolin) were used for prophylaxis. After sternotomy, bone wax was applied in the W group. Mediastinal and pericardial blood loss was collected into the waste container during the operation and measured. Skeletonized preparation of the LITA was done; side branches were clipped and cut with scissors. Simultaneously the saphenous vein was harvested. After LITA harvesting the patients were heparinized (100 IU/kg); the activated clotting time (HEMOCHRON International Technidyne Corporation, Edison, USA) was kept at 300 s throughout the operation and was neutralized completely with protamine after completion of the anastomoses. Once the target region for the anastomosis was identified, silicon loops (Quest Medical, Inc, Allen, TX) were placed proximally in all patients. For myocardial stabilization a mechanical stabilizer (Octopus; Medtronic, Inc, Minneapolis, MN) was applied. After arteriotomy the silicon loop was slightly elevated to avoid excessive bleeding during the introduction of the intraluminal shunt. The size of the shunt (Guidant Axius, Boston Scientific, Santa Clara, CA) was determined according to the target vessel diameter by the surgeon; oversizing was avoided. The shunt was removed prior to completion of the anastomosis. The anastomosis was done with running 7-0 polypropylene suture (Prolen, Visi-black, Ethicon). Good visibility was accomplished with a surgical blower (Axius Blower/Mister, Maquet Cardiovascular LLC, Wayne, NJ). The pericardium and mediastinum were drained in a standard fashion. The sternum was usually sutured with 8 wires, and one collagen-gentamicin sponge was inserted between the sternal halves. In patients with body weight from 90 to 100 kg one wire was added, but no more than 10 wires in patients exceeding 100 kg were used. We aimed to keep the hemoglobin level at about 10 g/dl during hospital stay.
Statistical analysis
Statistical analysis was performed using NCSS 2021 Statistical Software (2021) (NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/ncss); an associated probability of less than 0.05 was considered significant. Categorical data were analyzed by Fisher’s exact test. The Mann-Whitney U-test, Kolmogorov-Smirnov test and Student’s two-sample t-test were used for quantitative data. The variables in tables are expressed as the mean ± standard deviation (SD) or as the median and interquartile range. In accordance with Nguyen’s experience we performed a multivariate logistic regression analysis to define independent risk factors of blood loss at the threshold of around 800 ml. As this analysis did not reveal any significant difference, we recalculated it with a threshold of 750 ml. The outcomes were reported as odds ratios (OR) and corresponding 95% confidence intervals (CI).
Results
In the years 2013–2019, 661 OPCABs were performed at our institution. Of those, 58 patients were eligible candidates and were enrolled in the OPCAB study. Three patients from the NW group were excluded from the analysis post-hoc: 2 patients received only a single bypass on the left anterior descending artery (LAD). The third patient underwent emergent reexploration with ECC use for massive bleeding from the side branch of the LITA on the first pooperative day. Preoperative data did not show any differences between the groups (Table I).
Table I
Preoperative data
| Parameter | NW group (n = 26) | W group (n = 29) | P-value |
|---|---|---|---|
| Operation time* [min] | 186.2 ± 31.2 | 176.3 ± 23.9 | 0.19 |
| Intraoperative blood losses† [ml] | 750; 500–1025 | 550; 425–650 | 0.07108 |
Intraoperative results
The perioperative conversion rate to on-pump was zero. Intraoperative blood loss was 550 ml in the W group versus 750 ml in the NW group, but the differences between groups did not reach statistical significance (p = 0.0711) (Table II).
Table II
Intraoperative data
| Parameter | NW group(n = 26) | W group(n = 29) | P-value |
|---|---|---|---|
| Age* [years] | 68.6 ±7.25 | 67.3 ±7.8 | 0.53 |
| Body mass index* | 30.8 ±4.4 | 30.2 ±4.7 | 0.67 |
| Female sex, n (%) | 4 (15.3) | 5 (17.2) | 1.00 |
| Acetyl salicylic acid administration, n (%) | 24 (92.3) | 21 (72.4) | 0.08 |
| Ejection fraction†, % | 50; 45–60 | 55; 38–65 | 0.75 |
| EuroSCORE† | 1.42; 1.1–2.28 | 1.05; 0.77–2.2 | 0.08 |
| LMWH ≥ 12 hours preoperatively, n (%) | 9 (34.6) | 7 (24.1) | 0.39 |
| Preoperative hemoglobin† [g/dl] | 143; 122–152 | 142; 131–150 | 0.95 |
| Serum creatinine level† [μmol/l] | 93; 76–118 | 77; 68–96 | 0.13 |
Multivariate analysis for the risk of blood loss of ≥ 800 ml did not show any straightforward difference in our cohort. Therefore we re-calculated the analysis with the threshold of ≥ 750 ml. We identified absence (non-use) of bone wax (OR = 3.9, CI: 1.12–13.51, p = 0.027) and preoperative creatinin level (OR = 1.1, CI: 0.99–1.03, p = 0.03) as independent predictors of blood loss ≥ 750 ml.
Postoperative results
Early mortality and re-exploration due to bleeding were zero. Postoperative blood loss (p = 0.47) and the number of red blood cell units (p = 0.42) were similar in both groups. No procedure-related myocardial infarctions and no neurologic complications were observed. One-hunder percent 3-month follow-up was achieved. All patients were alive and no SWIs were recorded. Post-operative data are summarized in Table III.
Table III
Postoperative data
| Parameter | NW group(n = 26) | W group(n = 29) | P-value |
|---|---|---|---|
| 3 months mortality, n (%) | 0 | 0 | 1.00 |
| Myocardial infarction, n (%) | 0 | 0 | 1.00 |
| Stroke, n (%) | 0 | 0 | 1.00 |
| Transient ischemic attack, n (%) | 0 | 0 | 1.00 |
| Postoperative blood losses† [ml] | 625; 500–750 | 600; 500–750 | 0.47 |
| Wound infection, n (%) | 0 | 0 | 1.00 |
| Postoperative hemoglobin† [g/dl] | 94; 89–111 | 105; 92–113 | 0.14 |
| Red blood cells* [U] | 0.61 ±1.13 | 0.44 ±1.18 | 0.42 |
| Fresh frozen plasma [U] | 0 | 0 | 1.00 |
| Platelet concentrates [U] | 0 | 0 | 1.00 |
Discussion
Bone wax is still routinely used in cardiac surgery, but many controversial reports exist in the literature regarding its impact on blood loss or impaired wound healing. Bhatti et al. found in an animal study that bone wax reduces the inoculum of Staphlococcus aureus required to cause osteomyelitis, and that bone wax is still present in large quantities 4 weeks post-operatively. The authors advise against the liberal use of bone wax [4, 5]. In contrast, Prziborowski et al. in a large study did not confirm negative effects of bone wax on the percentage of postoperative wound complications [1]. A positive effect, such as a reduction of blood product substitution, was also not observed. They concluded that using bone wax is obviously safe but not particularly beneficial. Ozdemir et al. concluded from the analysis of postoperative drainage and blood transfusion requirement that bone wax does not reduce bleeding on sternal sides and does not affect sternal healing [2].
Any comparison of our results with the above-mentioned studies regarding the hemostatic effect of bone wax remains difficult. Their conclusions are based on the analysis of on-pump patients. To our knowledge there are no published results in the literature regarding the hemostatic benefit of bone wax by means of measurement of intraoperative blood loss in OPCAB. Difference in blood loss between our groups did not achieve statistical significance, although implementation of bone wax may reduce the risk of high intraoperative blood loss. Furthermore, there was low and comparable red blood cell consumption in both groups, probably due to good tolerance of moderate anemia. Reduced need for blood transfusion has become an important goal in cardiac surgery. Nowadays the risk of transfusion is not related to viral infection, but decreased survival probably results from reduced immunity in transfused patients [6, 7]. It can lead to a different strategy including the introduction of a cell saver into clinical practice. Nguyen in his OPCAB study used the autotransfusion set when the volume of collected blood reached the threshold of 800 ml (30.9% of patients). The threshold was chosen based on his previous clinical experience. Below this volume, the volume of treated blood is less than the blood volume contained in a packet of red blood cells, and does not justify the expense of its use. In addition he considered its usage useless in most patients who receive less than 4 grafts [8]. By contrast, in developing OPCABs in some institutions the autotransfusion system has been used routinely [9]. With regard to our data mentioned above, a question arises of both economic and medical constraints of cell-saver use in a limited number of grafted vessels. If the institutional standard is to employ a cell saver only in procedures with higher expected blood loss, the use of bone wax may reduce the risk of higher blood loss (above 750 ml) and thus result in an economic profit based on reduced cell-saver utilization.
In our study we observed that elevated preoperative serum creatinin level was also associated with a higher intraoperative blood loss. This was also seen in a study of Winkelmayer et al. and a systematic review and meta-analysis by Acedillo et al. where chronic kidney disease led to significantly higher intraoperative bleeding [10, 11]. The impact on postoperative bleeding requiring reexploration was not observed.
We noted a slight difference between groups in preoperative aspirin use (72.4% in W vs. 92.3% in NW group), although it did not reach statistical significance. Discontinuation of aspirin is not required before coronary surgery at our institution. Some patients did stop using aspirin preoperatively for various reasons (non-compliance, misunderstanding of invitation letter, etc.). We considered this influence not significant with regard to the study outcomes.
The incidence of deep sternal wound infection (DSWI) after cardiac surgery ranges from 0.7% to 3% [12]. However, as reported by Friberg et al., the incidence of sternal wound infection (SWI) can be much higher after discharge. Local collagen-gentamicin sponge insertion prevents sternal wound complications in patients with diabetes and chronic obstructive pulmonary disease [13]. Although only diabetic patients with higher risk of impaired wound healing were enrolled in our study, SWIs were not observed.
Limitations of the study: Our conclusions are based on a small cohort of patients and a limited number of grafted vessels. In general, a larger study population is required to obtain more accurate results. HbA1c was not routinely measured preoperatively to better assess longer term control of diabetes.
Conclusions
The use of bone wax does not lead to a higher risk of sternal wound infection. Its protective effect on intraoperative blood loss remains questionable. It may reduce the risk of high intraoperative blood loss, thus avoiding the need to use a cell saver during off-pump coronary surgery, leading to a cost benefit.