Introduction
Gastric cancer is the third most deadly cancer worldwide and accounts for around 8.3% of total deaths occurred due to cancer [1, 2]. It is a cancer of the uppermost intestinal tract, and within this, malignant tumorous cells develop in the lining of the stomach due to stomach infections, smoking, age, or dietary factors [3, 4]. Due to this, the patients suffer from indigestion, heartburn, nausea, fatigue, stomach pain, loss of appetite, and vomit blood or pass blood in stools [4, 5]. The survival rate with gastric cancer is low, so it must be diagnosed early via endoscopy and once detected immediately treated via surgery, medications, and chemotherapy [6]. Among these treatments, its main restorative treatment is surgical resection or gastrectomy. A minimal invasive and videoscopic surgical procedure is widely used these days to treat benign gastric cancer because surgery is still its only curative treatment; however, during surgery the extent of resection and lymphadenectomy that is needed must be considered carefully [7]. Minimally invasive surgical approaches are preferred because they demonstrate safety, feasibility, and oncologic equivalency more precisely as compared to conventional open gastrectomy [8]. Before surgery, it is essential to boost the immunity of the patient undergoing surgery, to reduce the postoperative infection and non-infectious difficulties and amend the prognosis of patients suffering from gastrointestinal cancer. For this purpose, the use of enteral immunonutrition (EIN), which is the enteral feeding formula of immunity boosting nutrients, is mainly recommended. The main components of EIN are ω-3 fatty acids, glutamine, arginine, and nucleotide, which primarily activates the immune system of the host [9, 10]. For example, Song et al. 2015 [11], Nikniaz et al. 2017 [12], and Cheng et al. 2018 [13] reported in their systematic review and meta-analysis that early administration of EIN in patients was more effective in improving their immunity index and post-surgical nutritional status. Similarly, Fu et al. 2021 [14] and Shen et al. 2022 [15] mentioned in their systematic review and meta-analysis that pre-operative use of EIN was effective and safe in reducing the post-operative infections, overall complications of surgery, and the duration of hospital stay of patients after surgery. These systematic reviews and meta-analysis are based on the results of various randomized controlled trials, and retrospective and prospective studies that were conducted to evaluate the potential benefits of EIN in patients undergoing gastrectomy. For instance, Guo et al. 2002 [16] reported in their prospective study that EIN helped in the fast recovery of patients. Similarly, Farreras et al. 2005 [17], Chen et al. 2005 [18], and Fujitani et al. 2012 [19] reported in their randomized controlled trials that EIN improved the defence mechanism and lowers the morbidity rate. Lee et al. 2016 [20] also reported the benefits of EIN in their retrospective study. Di Renzo et al. 2019 [21] found in their randomized controlled trials that EIN significantly improved the metabolic parameters of patients undergoing surgery. Likewise, Luo et al. 2019 [22], Claudino et al. 2019 [23], D’ Ignazio et al. 2020 [24], Sun et al. 2021 [25], Izumi et al. 2022 [26], and Xiao et al. 2022 [27] reported in their research studies that preoperative use of EIN boosted the immune response and significantly improved the serum prealbumin level and decreased postoperative morbidity in patients undergoing gastrectomy. However, although there are many studies that recommend the use of EIN for gastric cancer patients, still some like the studies of Bertolini et al. 2003 [28] and Mueller et al. 2022 [29] reported that the use of EIN was associated with sepsis and post-operative infections and caused more harm in gastric cancer patients than good. Nakamura et al. 2009 [30] reported that EIN was good only if provided at an optimal dose, otherwise its use was risky and hazardous. Hence, considering these contradictory results, we systematically reviewed the different studies related to the use of EIN for gastric cancer patients to evaluate their success and efficiency in boosting the immunity and improvement of overall survival rate.
Aim
This meta-analysis aims to evaluate the success of EIN in boosting the immunity of gastric cancer (GC) patients undergoing surgery.
Material and methods
In the current investigation, with the registration number YH/IRB/2022/786, we followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) normative criteria.
Search strategy
This meta-analysis is based on an extensive search conducted in the databases of Medline (via PubMed), Cinahl (via Ebsco), Scopus, and Web of Sciences from the year 2000 to 2022 using the following keywords: gastric cancer, gastrectomy, EIN, nutrition, immunity, surgery, randomized controlled trial, open-label study, prospective study, and retrospective study. Articles were included as per the PRISMA guidelines, and studies were selected as per the PICOS criteria randomly, irrespective of the language or type of study (randomized clinical trial, open-label study, prospective study, or retrospective study). Two authors (SR and LL) separately scanned the relevant sources for related studies. The full-text articles of the sources were collected, and abstracts were used only if they had sufficient information for the meta-analysis. Obsolete references were excluded, and useful studies were included as per the inclusion criteria. Two researchers (HL and SZ) independently retrieved a demographic description of the patients and event data with meaningful variables from the included studies [16–27].
Inclusion and exclusion criteria
Among the studies were those that reported on the use of EIN for gastric cancer patients undergoing surgery, as well as its comparison to other nutritional supplements. The studies were chosen between the years 2000 and 2022. We only included publications with the entire text and enough data for 2 × 2 tables in our investigation, while abstracts, studies with inadequate data, and relevant studies published before 2000 were eliminated.
Analytical standard evaluation and source of heterogeneity
Two reviewers (HL and SZ) independently assessed the methodological validity of the included papers, and the heterogeneity of the included experiments was estimated. Author LL oversaw mediation of any disagreements between authors (HL and SZ). Cochran statistics were used to study heterogeneity, and the I2 index in random bivariate mode was determined using RevMan [31] and MedCalc software [32]. The use of randomized controlled trials vs. open-label studies, retrospective vs. prospective research, varying numbers of patients with different stages of gastric cancer, and the use of different nutritional supplements for cancer patients were the origins of the observed heterogeneity.
Statistical analysis
RevMan and MedCalc software were used to conduct a meta-analysis. The DerSimonian Lair technique was used to generate the diagnostic odds ratio and risk ratio for statistical analysis, using a 2 × 2 table created with the event data. Statistical factors such as odds ratio and risk difference were determined, and forest plots were generated using RevMan software. The χ2 value, τ2 value, df value, I2 value, z-value, and p-value were used to assess study heterogeneity. The risk of bias graph and summary were created using RevMan software, and publication bias was analysed using Begg’s test, Egger’s test, and Deek’s funnel plot [33] using MedCalc software.
Results
Literature search results
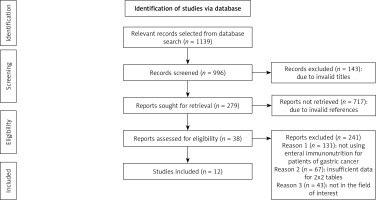
We found a total of 1139 studies through electronic scans from different databases as per the PICOS criteria [34] shown in Table I. Among these studies, we excluded 143 studies by reading their titles and abstracts, and 996 records were screened. Further, due to invalid references and duplicity, we excluded 717 studies and included only 279 studies for final screening. Out of these 279 studies, 241 studies were excluded based on the inclusion criteria, and the eligibility of the remaining 38 studies was assessed further. The key reasons for omission were inadequate evidence and inappropriate comparison criteria to create 2 × 2 tables for review. Finally, for meta-analysis, 12 studies ranging from the years 2000 to 2022 fulfilled the inclusion criteria, i.e. the use of EIN for gastric cancer patients was used as shown in Figure 1. A total of 10,422 gastric cancer patients of various ages were included in the studies. These patients were chosen randomly and provided with either EIN or other immunity-boosting supplements before gastrectomy. The demographic details of the studies included in this meta-analysis are shown in Table II. It describes the author of the study, publishing year, type of study, the intervention of the study, total sample size, age of patients, type of supplements used, number of patients selected for the trial, results of the study, and p-value. Later, these event data were used to perform the meta-analysis.
Table I
PICOS search
Table II
Demographic summary of included studies
| Study ID and year | Journal of publication | Type of study | Intervention | Sample size | Age of patients | Control group | Intervention group | Results | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Guo et al. 2002 [16] | Journal of Gastroenterology and Hepatology | Prospective study | Screening of the nutritional risk of patients with gastric carcinoma | 314 | 30–70 years | 125/314 | 190/314 | Preoperative nutritional support is necessary for fast recovery of patients | < 0.05 |
| Farreras et al. 2005 [17] | Clinical Nutrition | Randomized clinical trial | Effect of early postoperative enteral immunonutrition in patients undergoing gastric cancer | 66 | 18–60 years | 30/66 | 36/66 | Patients receiving the supplemented formula showed significantly better wound healing and low morbidity. | 0.005 |
| Chen et al. 2005 [18] | Asian Journal of Surgery | Prospective randomized clinical trial | Role of enteral immunonutrition in gastric cancer patients undergoing surgery | 40 | 31–75 years | 20/40 | 20/40 | Enteral immunonutrition improved the defence mechanisms and modulated the inflammatory action | < 0.01 |
| Fujitani et al. 2012 [19] | The British Journal of surgery | Randomized controlled trial | Effect of preoperative enteral immunonutrition on patients undergoing gastrectomy for gastric cancer | 244 | 26–78 years | 117/244 | 127/244 | Enteral immunonutrition reduced the morbidity rate | < 0.05 |
| Li et al. 2016 [20] | Medicine | Retrospective Analysis | Assessment of clinical significance of the Prognostic Nutritional Index for Gastrectomy | 7781 | 45–80 years | 779/7781 | 7002/7781 | PNI is a useful predictor for patients at high risk of postoperative morbidity and mortality | < 0.001 |
| Di Renzo et al. 2019 [21] | European Review for Medical and Pharmacological Sciences | Randomized clinical trial | Evaluation of the effect of an immuno-enhanced formula in gastric cancer patients | 50 | 18–80 years | 13/44 | 31/44 | EIN treatment showed a significant improvement in metabolic parameters in gastric cancer patients | < 0.05 |
| Luo et al. 2019 [22] | European Journal of Surgical Oncology | Multicentre retrospective study | Prognostic impact of preoperative nutritional index (PNI) on advanced gastric cancer | 50 | 25–70 years | 13/50 | 25/50 | Low PNI results in poor recurrence-free survival and overall survival of patients | < 0.001 |
| Claudino et al. 2019 [23] | Nutrition | Retrospective study | Evaluation of the rate and survival of patients with gastric cancer undergoing immunonutrition | 164 | 18–80 years | 56/164 | 108/164 | Study confirmed the benefit of immunonutrition supplementation for overall survival of patients | < 0.001 |
| D’ Ignazio et al. 2020 [24] | Clinical Nutrition ESPEN | Prospective pilot study | Impact of enteral immunonutrition on the cell-mediated immune response in gastric cancers. | 24 | 44–90 years | 8/24 | 16/24 | Enteral immunonutrition activated the immune response in patients of gastric cancers | < 0.005 |
| Sun et al. 2021 [25] | BMC Gastroenterology | Retrospective study | Effect of Controlling Nutritional Status (CONUT) Score in gastric cancer | 1479 | 40–80 years | 431/1479 | 1048/1479 | CONUT is an important factor for postoperative complications in both early and advanced stage gastric cancer | < 0.001 |
| Izumi et al. 2022 [26] | World Journal of Surgery | Original scientific report | Change in Serum Prealbumin Level after Preoperative Enteral Nutrition in patients of Gastric Cancer | 50 | 18–70 years | 10/50 | 40/50 | Preoperative enteral nutrition improved the serum prealbumin level and decreased postoperative morbidity in gastric cancer patients. | < 0.005 |
| Xiao et al. 2022 [27] | European Journal of Clinical Nutrition | Retrospective study | Study of the association between prognostic nutritional index (PNI) on post-operative infection and prognosis of gastric cancer patients | 160 | 40–80 years | 633/2352 | 1719/2352 | Low PNI is associated with poorer overall survival | 0.001 |
Meta-analysis results
The meta-analysis was performed using RevMan and MedCalc software. The results are discussed below.
Bias risk assessment
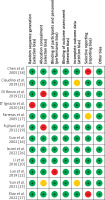
The risk of bias for included studies was assessed as shown in Table III. The risk of bias graph (Figure 2) and risk of bias summary (Figure 3) show that the current meta-analysis has a low risk of bias. Publication bias was measured with the help of Egger’s test, Begg’s test, and Deek’s funnel plot. The current meta-analysis has a low risk of publication bias, as is apparent from the funnel plot shown in Figure 4, and the p-values of both tests are significant because they are greater than 0.05 [35]: Egger’s test p-value is 0.365 and Begg’s test p-value is 0.453.
Table III
Risk assessment for included studies
| Study ID and year | Was a consecutive or random sample of patients enrolled? | Did the study avoid inappropriate exclusions? | Did all patients receive the same reference standard? | Were all patients included in the analysis? | Was the sample frame appropriate to address the target population? | Were the study participants sampled in an appropriate way? | Were the study subjects and the setting described in detail? | Were valid methods used for the identification of the condition? | Was the condition measured in a standard, reliable way for all participants? | Was there appropriate statistical analysis? |
|---|---|---|---|---|---|---|---|---|---|---|
| Guo et al. 2002 [16] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Farreras et al. 2005 [17] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Chen et al. 2005 [18] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Fujitani et al. 2012 [19] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Li et al. 2016 [20] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Di Renzo et al. 2019 [21] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Luo et al. 2019 [22] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Claudino et al. 2019 [23] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| D’ Ignazio et al. 2020 [24] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Sun et al. 2021 [25] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Izumi et al. 2022 [26] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Xiao et al. 2022 [27] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
Statistical assessment
The diagnostic odds ratio and risk ratio of the included studies were calculated using RevMan software, and the respective forest plots were designed as shown in Figures 5 and 6, respectively. We obtained an odds ratio value of 0.23 (95% CI: 0.09–0.59). The odds ratio value of less than 1 is suggestive of the high likelihood of EIN boosting the immunity of patients and the overall survival rate with a low risk of morbidity and mortality. The results are heterogeneous with a τ2 value of 2.77, a χ2 value of 1707.96, a df value of 11, an I2 value of 99%, a z value of 3.04, and a p-value less than 0.05. The risk ratio is 0.47 (95% CI: 0.29–0.77) with heterogeneity of τ2 value of 0.73, χ2 value of 1428.34, df value of 11, I2 value of 99%, z value of 2.99, and p-value of less than 0.05. The risk ratio value, which compares the risks of supplements used in the 2 groups, is also less than 1, which indicates that the use of EIN for gastric cancer patients undergoing surgery is safe and effective. The I2 value greater than 50% indicates the high heterogeneity [36] and random effect model for meta-analysis. All these results are statistically significant with a p-value less than 0.05 and are indicative of the high efficiency of EIN in boosting the immunity of gastric cancer patients undergoing gastrectomy.
Discussion
“Cancer is the most dreadful and fatal disease and accounts for around 10 million deaths a year.” [37]. Among the different cancers, gastric cancer is the most common and third most deadly cancer reported worldwide. Due to failure of genetic control, uncontrolled cell division occurs, and abnormal malignant tumours form in the upper intestinal tract. As cancer has the peculiar characteristic of metastasis, these tumour cells of the uppermost intestinal region spread very fast via blood to all other parts of the body, causing multiple organ failures and, if not treated promptly, death [36, 38]. Because the invasion speed of cancer is very fast, its primary treatment is the removal of the cancerous tumour via resection surgery or gastrectomy [39–41]. Although surgery is the best way to treat a cancer patient, the major risks associated with this are post-operative infections, surgical complications, longer duration of hospital stay, morbidity, and mortality [42, 43]. Currently, endoscopic mucosal resection surgery is commonly preferred because it is an effective treatment modality with comparable results to that of conventional surgery [44]. Uyama et al. 2013 [45] mentioned that endoscopic submucosal dissection (ESD) reduces the local recurrence rate and is the best surgical resection process along with regional lymphadenectomy for radical gastrectomy. Similarly, Zhang et al. 2021 [46] recommended laparoscopic gastrectomy (LG) as an emerging surgical approach that has significant advantages in short-term outcomes as compared to the open surgical procedures for patients of gastric cancer. Robot-assisted (RAGD2) and laparoscopy-assisted gastrectomy with D2 lymphadenectomy (LAGD2) is specifically preferred for patients with gastric cancer because they reduce operative blood loss and postoperative complications. Hence, to reduce these infections and complications, it is essential to boost the immunity of patients by providing them with immunity-enhancing supplements, and for this, EIN is mostly recommended [47].
As shown in Figure 7, EIN is an enteral feeding formula made up of nutritional supplements such as amino acids arginine and/or glutamine, omega-3 predominant marine oils, nucleic acids, antioxidants, vitamins, and minerals [48]. These supplements are used because amino acids cause immune cell activation by improving cell metabolic reprogramming via receptor ligation and by enhancing the rate of transcription and translation [49]. Similarly, fatty acids and nucleic acids are important for the acquisition of biomass for cell division and cytokine and immune cell production [50]. Antioxidants like selenium, vitamin C, and vitamin E help to prevent the oxidative damage of cells during the immune response to invading pathogens and infectious agents [51]. Due to these beneficial aspects, various studies have recommended the use of EWIN as an effective preoperative supplement for patients undergoing gastrectomy; Wong et al. 2016 [52] reported in their systematic review and meta-analysis that the use of EIN enhances the immune defence system and reduces the duration of hospital stay, obtaining a lower risk ratio (RR) of 0.59 with a 95% confidence interval (CI) of 0.40 to 0.88. In the same way, the systematic review of Heyland et al. 2001 [53] recommended EIN for people with gastric cancer.
Similarly to these studies, in our meta-analysis, with statistically significant results (p < 0.05) and an odds ratio value of 0.23 (95% CI: 0.09–0.59), i.e. less than 1, and a risk ratio value of 0.47 (95% CI: 0.29–0.77), i.e. less than 1, we conclude that pre-operative and early use of EIN is safe and effective for boosting the immunity of gastric cancer patients undergoing surgery.
The limitations of the present study are the variability of nutritional supplements used and the evaluation of metabolic and immunological parameters via different tests performed by different laboratory technicians, which in turn influences the risk of false-negative results. Data from other relevant studies that show potential benefits of EIN in boosting the defence mechanisms can also include more details about patients’ health status and behaviour to indicate the importance and efficiency of these studies more clearly. To see the variability, detailed data on the patients’ case histories, physical examinations, and pathological tests can further support the safety and efficacy of EIN in boosting the immunity of gastric cancer patients undergoing resection surgery.
Conclusions
Gastric cancer is the most frequently reported cancer of the uppermost intestinal tract and is fatal if not treated promptly. Because its primary and most effective treatment is surgery or gastrectomy, it is essential to boost the immunity of patients undergoing surgery to avoid post-operative infections, complications, morbidity, and mortality and to increase their overall survival rate. For this purpose, EIN is the commonly recommended enteral feeding supplement, but some studies have reported its risks and adverse effects too. Thus, to address these issues, we conducted this systematic review and meta-analysis to assess its safety and efficiency in boosting the immunity of gastric cancer patients undergoing gastrectomy. Based on statistically significant results (p < 0.05), we highly recommend the preoperative and early use of EIN for boosting the immunity of gastric cancer patients undergoing surgery.