Introduction
Surgical site infections (SSIs) occur after an operative procedure and can range from superficial to deep wound infections. Global estimates of SSIs have ranged from 0.5% to 15%, whereas studies in India have consistently shown higher rates from 23% to 38% [1]. SSIs are a substantial cause of morbidity, prolonged hospitalization, hospital readmissions, and death and pose a considerable financial burden on healthcare systems [2, 3]. Thus, prevention and minimization of SSIs improve patient outcomes and reduce resource consumption [4, 5].
Strategies to reduce the risk of SSIs include interventions that can be delivered preoperatively, intraoperatively, or postoperatively. The World Health Organization (WHO) and the Centers for Disease Control (CDC) have proposed guidelines recommending measures to prevent SSIs [6–8]. Sterile procedures, maintaining patient homeostasis, wound closure interventions, and prophylactic antibiotics are commonly used to reduce SSI risk [9]. Intraoperative measures primarily focus on decontamination of the skin and intraoperative wound irrigation using soap and antiseptics and are a simple, efficient, and cost-effective measure to reduce SSIs [10]. The most frequently used antiseptic is povidone-iodine (PVI), commonly applied as irrigation or a spray. PVI is an iodophor in which iodine is complexed with the polymer povidone. The microbicidal activity of iodine involves inhibition of vital bacterial cellular mechanisms and structures [11].
In contrast to antibiotics, antiseptics have a broader spectrum of activity and a reduced likelihood of resistance. However, despite the potential usefulness of topical antiseptics, current clinical practice is variable and largely dependent on surgeon preference. Furthermore, the routine use of topical antibiotics and antiseptics has been associated with adverse effects such as tissue toxicity and interference with wound healing [12, 13].
Although systematic reviews and meta-analyses on the benefits of PVI in reducing the incidence of SSIs have been published, there has been no definite conclusion on the effectiveness of PVI in different surgical categories [10, 14–16].
Aim
The objective of this paper is to synthesize current evidence from available randomized controlled trials evaluating the efficacy of PVI vs. saline/no treatment controls in decreasing the incidence of SSI.
Material and methods
The meta-analysis was carried out using the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) normative recommendations in this study with the registration number LPH#/IRB/2021/1025.
Informed consent was not taken because of meta-analysis nature of this study.
Search strategy
A systematic literature search was conducted of MEDLINE (PubMed) and Cochrane Register of Controlled Trials (CENTRAL) in June 2021. No time limit was applied as several studies were published earlier than 1990. The following search terms were used in various combinations: surgical site infection, wound infection, SSI, post-operative, povidone-iodine, betadine, irrigation, and spray, and lavage, intraoperative and anti-infective agents. Additionally, a comprehensive list of search terms, including Medical Subject Headings (MeSH) terms, was applied. The titles and abstracts of potentially relevant studies were scanned, and the full-text versions of the relevant articles were read. Additional studies were identified by cross-checking the reference lists of the relevant studies.
Study selection or inclusion/exclusion criteria
Randomized controlled studies (RCTs) and prospective randomized studies that compared povidone-iodine application in any form (irrigation, spray, lavage, scrub) either preoperatively or intraoperatively were included across various surgical categories. The comparator treatment in the studies was primarily saline or no treatment. All studies reporting SSIs or wound infections as outcomes were included irrespective of the definition of SSI used. Exclusion criteria were non-randomized studies, animal studies, and studies with bacteriological counts as endpoints.
Data extraction and quality assessment
Following identification of articles that met the inclusion criteria, data were extracted using a predefined data extraction form that included the following items: study author, publication year, surgery category, inclusion criteria, intervention, control, SSI data in each group, PVI administration method, follow-up time, type of procedure, any systemic or prophylactic antibiotic use and other comments (if necessary).
The Cochrane Collaboration’s risk of bias tool was used to assess the methodological quality of the included studies [17]. This tool includes the following criteria: randomization, allocation concealment, blinding, and completeness of follow-up. In addition, the risk of bias for each item was graded as high, low, or unclear risk.
Statement of ethics
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable standards. Informed consent was not required due to the meta-analysis nature of this study. This study was approved by the Research Ethics Committee of Liyang People’s Hospital with the registration number of LPH#/IRB/2021/1025.
Quantitative data synthesis
Meta-analysis was performed using Review Manager (RevMan, Version 5. Copenhagen: The Nordic Cochrane Center, the Cochrane Collaboration. 2020). Absolute numbers of participants in each study developing a wound infection or SSI and the total number of participants in each group (intervention and control group) were used to calculate the risk ratio and the 95% confidence interval (CI). Meta-analyses were done using a random-effects model (Mantel-Haenszel method), and heterogeneity in the included studies was evaluated using the I2 statistic, with small heterogeneity for I2 values of 25%, moderate heterogeneity for I2 values of 25% to 50%, and high heterogeneity for I2 values > 50% [18]. Forest plots were constructed, and p < 0.05 was statistically significant. Subgroup analyses were also performed according to the type of comparator, PVI administration method, surgery category, and type of procedure.
Publication bias was assessed by a funnel plot in which the log risk ratio for each study was plotted against its standard error. Egger’s and Begg’s tests were performed using Comprehensive Meta-Analysis (Version 3.3.070) [19].
Results
Identification of studies
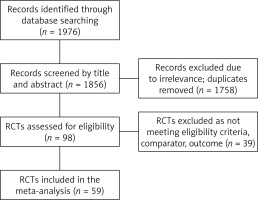
A total of 1976 records were identified by database searching, of which 1856 were screened by title and abstract. Duplicates and irrelevant records were removed (n = 1758), and 98 RCTs were assessed for eligibility. However, 39 RCTs were excluded due to reasons such as inappropriate comparator (other antiseptic or PVI of different concentrations), duplicate data, use of antiseptics other than PVI, or absence of information on SSI or wound infection as an outcome. The process of selection is shown in Figure 1.
Study characteristics
Fifty-nine RCTs totaling 20,497 participants met the inclusion criteria (PVI intervention group: 10148 participants and control group: 10349 participants). These RCTs involved the comparison of PVI intervention to saline or no treatment control groups across various surgical categories. All studies were randomized controlled trials or prospective randomized studies with sample sizes ranging from 29 to 3027. The studies included male and female participants undergoing various elective and urgent surgical procedures.
In 34 of the selected studies, PVI was administered as an irrigation solution, and it was given as a dry powder spray in 13 studies. The comparators in the studies were no treatment or propellant only and saline. The concentration of PVI ranged from 0.35% to 10%. Table I shows the characteristics of the intervention and control groups of the studies included for meta-analysis [20–78].
Table I
Characteristics of intervention and control groups of the included studies
| Reference | Intervention | Control | Type of PVI administration |
|---|---|---|---|
| Anderson 2020 [20] | PVI | None | Irrigation |
| Alobaidy 2020 [21] | PVI | None | Irrigation |
| Aref 2018 [22] | 10% PVI solution | None | Irrigation |
| Asad 2017 [23] | PVI solution | None | Irrigation |
| Asghania 2011 [24] | 10% PVI solution | None | Scrub |
| Barat 2016 [25] | 10% PVI solution | None | Irrigation |
| Barr 1978 [26] | PVI | None | Lavage |
| Calkins 2019 [27] | Dilute Betadine and 10% Betadine | Saline | Lavage |
| Chang 2006 [28] | 0.35% PVI + 2000 ml normal saline | 2000 ml normal saline | Irrigation |
| Charoenviboonphan 2011 [29] | 1% PVI paint | None | Paint |
| Cheng 2005 [30] | 0.35% PVI + 2000 ml normal saline | Saline | Irrigation |
| Cohen 2020 [31] | 0.35% PVI | Saline | Irrigation |
| de Jong 1982 [32] | 1% and 10% PVI solution | None | Irrigation |
| Foster 1981 [33] | PVI dry powder | None | Spray |
| Galland 1977 [34] | PVI dry powder | None | Spray |
| Galland 1983 [35] | PVI aerosol | None | Spray |
| Galle 1980 [36] | PVI solution | Saline | Irrigation |
| Ghafouri 2016 [37] | 1% PVI solution | Saline | Irrigation |
| Gilmore 1974 [38] | PVI dry powder | None | Spray |
| Gilmore 1975 [39] | PVI dry powder | Propellant alone | Spray |
| Gilmore 1977 [40] | PVI dry powder | Propellant alone | Spray |
| Gray 1981 [41] | PVI dry powder | None | Spray |
| Guzman 2002 [42] | PVI solution | Saline | Irrigation |
| Haas 2010 [43] | 1% PVI solution | None | Scrub |
| Haider 2018 [44] | 1% PVI solution | None | Irrigation |
| Harihara 2006 [45] | PVI solution | Saline | Irrigation |
| Hassan 2016 [46] | 10% PVI solution | Saline | Irrigation |
| Iqbal 2015 [47] | 1% PVI solution | None | Irrigation |
| Johnson 1985 [48] | 50 ml of 1% PVI | Saline | Irrigation |
| Karuserci 2019 [49] | 10% PVI + saline | Saline | Irrigation |
| Ko 1992 [50] | 0.5% PVI solution in saline | Saline | Irrigation |
| Kokavec 2008 [51] | 0.35% PVI solution | Saline | Irrigation |
| Kothi 1981 [52] | PVI solution | None | Irrigation |
| Lau 1986 [53] | 10 ml 1% PVI solution | None | Irrigation |
| Mahomed 2016 [54] | 50 ml 10% PVI solution | None | Irrigation |
| Memon 2011 [55] | 10% PVI | None | Scrub |
| McCluskey 1976 [56] | 10% PVI solution | None | Irrigation |
| Morgan 1978 [57] | PVI spray | None | Spray |
| Naunton Morgan 1980 [58] | PVI dry powder | None | Spray |
| Muller 2018 [59] | 1000 ml PVI solution | 1000 ml Ringer’s lactate solution | Irrigation |
| Mwangi 2013 [60] | PVI solution | None | Irrigation |
| Nandi 2015 [61] | PVI | None | Scrub |
| Oestreicher 1989 [62] | 10% PVI solution | Saline | Irrigation |
| Olmez 2013 [63] | PVI solution | None | Irrigation |
| Parker 1985 [64] | 10% PVI solution | Water | Lavage |
| Pollock 1978 [65] | PVI spray | Sterile saline | Spray |
| Reid 2001 [66] | 10% PVI solution | None | Scrub |
| Rogers 1983 [67] | 10% PVI solution | Normal saline | Irrigation |
| Sherlock 1984 [68] | PVI spray | None | Spray |
| Sindelar 1979a [69] | 0.1% PVI solution | Saline | Irrigation |
| Sindelar 1979b [70] | 10% PVI solution | Saline | Irrigation |
| Sindelar 1985 [71] | 1% PVI solution | Saline | Irrigation |
| Starr 2005 [72] | 5% PVI solution | None | Scrub |
| Stokes 1977 [73] | PVI spray | None | Spray |
| Vallance 1985 [74] | 100 ml PVI + saline | Normal saline | Lavage |
| Vinay 2019 [75] | 5% PVI solution | Normal saline | Irrigation |
| Walker 2013 [76] | 10% PVI solution | Saline | Gauze soaked |
| Walsh 1981 [77] | 0.5% Betadine spray | None | Spray |
| Yildirim 2012 [78] | PVI solution | None | Irrigation |
Characteristics of surgical interventions
Most studies were conducted in participants undergoing abdominal surgery (n = 26), gynecological procedures, specifically elective or urgent cesarean section (n = 17), and appendectomy (n = 8). Elective operations were performed in 25 studies, urgent operations in 12 studies, and mixed operations in 22 studies. The use of antibiotics was inconsistent between the studies but was administered in both intervention and control groups of the studies with prophylactic antibiotics (n = 21). Table II summarizes the characteristics of the surgical procedures and types of procedures included for quantitative synthesis.
Table II
Characteristics of included studies
| Reference | Surgery type | Type of procedure (Urgent/elective) | Sample size | Follow-up |
|---|---|---|---|---|
| Anderson 2020 [20] | Abdominal | Urgent | 100 | 1 year |
| Alobaidy 2020 [21] | Gynecological | Elective | 400 | NS |
| Aref 2018 [22] | Gynecological | Elective | 207 | NS |
| Asad 2017 [23] | Gynecological | Urgent | 434 | 3 weeks |
| Asghania 2011 [24] | Gynecological | Elective | 568 | NS |
| Barat 2016 [25] | Gynecological | Elective | 400 | 6 weeks |
| Barr 1978 [26] | Abdominal | Mixed | 88 | NS |
| Calkins 2019 [27] | Orthopedic | Elective | 457 | 90 days |
| Chang 2006 [28] | Spinal | Elective | 244 | 19 months |
| Charoenviboonphan 2011 [29] | Gynecological | Mixed | 599 | NS |
| Cheng 2005 [30] | Spinal | Elective | 414 | 15.5 months |
| Cohen 2020 [31] | Spinal | Elective | 153 | 30 days |
| de Jong 1982 [32] | Abdominal+mixed | Mixed | 582 | 4 weeks |
| Foster 1981 [33] | Abdominal | Urgent | 236 | 4 weeks |
| Galland 1977 [34] | Abdominal | Mixed | 78 | NS |
| Galland 1983 [35] | Abdominal | Urgent | 200 | 4 weeks |
| Galle 1980 [36] | Abdominal | 67 | NS | |
| Ghafouri 2016 [37] | Trauma | Urgent | 389 | NS |
| Gilmore 1974 [38] | Abdominal | Mixed | 300 | 4 weeks |
| Gilmore 1975 [39] | Abdominal | Mixed | 144 | 6 weeks |
| Gilmore 1977 [40] | Non-abdominal | Mixed | 101 | 6 weeks |
| Gray 1981 [41] | Abdominal | Elective | 153 | 2 weeks |
| Guzman 2002 [42] | Gynecological | Elective | 160 | NS |
| Haas 2010 [43] | Gynecological | Elective | 300 | 1 month |
| Haider 2018 [44] | General | Elective | 600 | 4 weeks |
| Harihara 2006 [45] | Gastric and colorectal | Elective | 107 | NS |
| Hassan 2016 [46] | Gynecological | Elective | 100 | NS |
| Iqbal 2015 [47] | Abdominal | Urgent | 166 | 30 days |
| Johnson 1985 [48] | Proctectomy for carcinoma | Elective | 56 | 3 months |
| Karuserci 2019 [49] | Abdominal | Mixed | 200 | 30 days |
| Ko 1992 [50] | Cardiopulmonary bypass | Mixed | 1980 | 30 days |
| Kokavec 2008 [51] | Orthopedic | Elective | 162 | 1.5 month |
| Kothi 1981 [52] | Abdominal | Elective | 220 | 2 weeks |
| Lau 1986 [53] | Abdominal | Urgent | 315 | 6 weeks |
| Mahomed 2016 [54] | Gynecological | Elective and Urgent | 3027 | 4 weeks |
| McCluskey 1976 [55] | Abdominal | Mixed | 110 | 4 weeks |
| Memon 2011 [56] | Gynecological | Mixed | 200 | NS |
| Morgan 1978 [57] | Accident trauma | Urgent | 320 | 6 days |
| Naunton Morgan 1980 [58] | Accident trauma | Urgent | 572 | NS |
| Muller 2018 [59] | Abdominal | Elective | 44 | 30 days |
| Mwangi 2013 [60] | Gynecological | Mixed | 397 | 2 weeks |
| Nandi 2015 [61] | Gynecological | Mixed | 294 | NS |
| Oestreicher 1989 [62] | Mixed | Mixed | 540 | NS |
| Olmez 2013 [63] | Gynecological | Mixed | 667 | NS |
| Parker 1985 [64] | Resection of bowel carcinoma | Elective | 45 | NS |
| Pollock 1978 [65] | Abdominal | Mixed | 139 | 4 weeks |
| Reid 2001 [66] | Gynecological | Elective | 430 | NS |
| Rogers 1983 [67] | Abdominal+mixed | Mixed | 187 | 4 weeks |
| Sherlock 1984 [68] | Abdominal | Urgent | 75 | 4 weeks |
| Sindelar 1979a [69] | Abdominal | Elective | 168 | NS |
| Sindelar 1979b [70] | General | Mixed | 500 | 12 weeks |
| Sindelar 1985 [71] | Abdominal | Mixed | 75 | NS |
| Starr 2005 [72] | Gynecological | Elective | 308 | NS |
| Stokes 1977 [73] | Abdominal | Urgent | 117 | NS |
| Vallance 1985 [74] | Abdominal | Mixed | 29 | 1 month |
| Vinay 2019 [75] | Abdominal | Elective | 180 | 30 days |
| Walker 2013 [76] | Vascular | Elective | 67 | 6 weeks |
| Walsh 1981 [77] | Abdominal | Mixed | 627 | 4 weeks |
| Yildirim 2012 [78] | Gynecological | Mixed | 669 | 6 weeks |
Bias assessment
The results of the risk of bias evaluation are shown in Figure 2. Overall, there was a high risk of bias due to unclear or high risk related to selection and performance bias and unclear risks associated with detection and reporting bias.
Figure 4
Forest plot of studies included in the meta-analysis (n = 59) using a random effects model. Risk ratios and 95% confidence intervals are shown
PVI – povidone iodine, control – saline or no treatment.

The funnel plot was asymmetrical (Figure 3), and Egger’s and Begg’s tests were statistically significant, indicating the possibility of publication bias.
Surgical site or wound infection rates
The incidence of SSI or wound infection in the included studies is shown in Table III. The SSI incidences ranged from 0% to 84.6% in the PVI group and from 0.6% to 75% in the control group (no treatment or saline). The overall incidence of SSI was 6.6% in the PVI intervention group and 9.4% in the control group.
Table III
Surgical site infection (SSI) or wound infection incidences in the included studies
| Reference | SSI incidence (%) | |
|---|---|---|
| Intervention: PVI | Control: saline or no treatment | |
| Anderson 2020 [20] | 12 | 16 |
| Alobaidy 2020 [21] | 0.5 | 1 |
| Aref 2018 [22] | 3.8 | 5.9 |
| Asad 2017 [23] | 1.4 | 3.7 |
| Asghania 2011 [24] | 3.5 | 3.2 |
| Barat 2016 [25] | 6 | 6.5 |
| Barr 1978 [26] | 3.6 | 38.3 |
| Calkins 2019 [27] | 0.4 | 3.4 |
| Chang 2006 [28] | 0 | 4.8 |
| Charoenviboonphan 2011 [29] | 0.3 | 1.3 |
| Cheng 2005 [30] | 0 | 3.4 |
| Cohen 2020 [31] | 1.3 | 2.6 |
| de Jong 1982 [32] | 12.9 | 16.1 |
| Foster 1981 [33] | 24.4 | 23.1 |
| Galland 1977 [34] | 35.9 | 46.2 |
| Galland 1983 [35] | 13.7 | 13.3 |
| Galle 1980 [36] | 29 | 25 |
| Ghafouri 2016 [37] | 7.7 | 7.3 |
| Gilmore 1974 [38] | 8.1 | 15.9 |
| Gilmore 1975 [39] | 8.6 | 24.3 |
| Gilmore 1977 [40] | 0 | 3.8 |
| Gray 1981 [41] | 9.9 | 24.4 |
| Guzman 2002 [42] | 8.8 | 5 |
| Haas 2010 [43] | 4.5 | 6.9 |
| Haider 2018 [44] | 6.3 | 8 |
| Harihara 2006 [45] | 14.8 | 15.1 |
| Hassan 2016 [46] | 2 | 14 |
| Iqbal 2015 [47] | 10.8 | 19.3 |
| Johnson 1985 [48] | 35.7 | 75 |
| Karuserci 2019 [49] | 6 | 12 |
| Ko 1992 [50] | 1.1 | 0.6 |
| Kokavec 2008 [51] | 0 | 2.7 |
| Kothi 1981 [52] | 15.7 | 12.7 |
| Lau 1986 [53] | 5.7 | 1.9 |
| Mahomed 2016 [54] | 9.5 | 9.8 |
| McCluskey 1976 [55] | 37.5 | 25.9 |
| Memon 2011 [56] | 1 | 3 |
| Morgan 1978 [57] | 6 | 14.3 |
| Naunton Morgan 1980 [58] | 5.3 | 14.6 |
| Muller 2018 [59] | 17.4 | 9.5 |
| Mwangi 2013 [60] | 6.5 | 10.2 |
| Nandi 2015 [61] | 2.9 | 5.1 |
| Oestreicher 1989 [62] | 6 | 5.5 |
| Olmez 2013 [63] | 10.5 | 17 |
| Parker 1985 [64] | 4.5 | 39.1 |
| Pollock 1978 [65] | 26.2 | 35.1 |
| Reid 2001 [66] | 5.5 | 8.5 |
| Rogers 1983 [67] | 4.7 | 10.9 |
| Sherlock 1984 [68] | 15.4 | 36.1 |
| Sindelar 1979a [69] | 1.3 | 10.2 |
| Sindelar 1979b [70] | 2.9 | 15.1 |
| Sindelar 1985 [71] | 2.7 | 7.9 |
| Starr 2005 [72] | 0.7 | 1.2 |
| Stokes 1977 [73] | 20 | 33.9 |
| Vallance 1985 [74] | 84.6 | 62.5 |
| Vinay 2019 [75] | 10 | 7.8 |
| Walker 2013 [76] | 3.2 | 8.3 |
| Walsh 1981 [77] | 9.1 | 12.5 |
| Yildirim 2012 [78] | 1.8 | 2.7 |
Meta-analysis results
The results of the meta-analysis for all the studies included (n = 59) showed a reduction in the incidence of SSI and wound infections with the application of PVI in any form across all surgical categories compared to saline treatment or no treatment controls, which was statistically significant (RR = 0.70, 0.60 to 0.80, p = 0.0002, I2 = 44%) (Figure 4).
Figure 6
Forest plot for subgroup analysis of PVI application method in studies using a random effects model. Risk ratios and 95 confidence intervals are shown
PVI – povidone iodine, control – saline or no treatment.

Stratification of the results by the type of comparator showed that the decrease in SSI incidence remained statistically significant for PVI vs. saline or no treatment control groups (Figure 5). The test for subgroup differences indicated no statistically significant subgroup effect (p = 0.63), suggesting that comparator type does not modify the effect of PVI. However, the heterogeneity was high (I 2 = 60%) in the saline comparator subgroup, suggesting inconsistency in the studies included.
Figure 7
Forest plot for subgroup analysis of surgery category in studies using a random effects model. Risk ratios and 95 confidence intervals are shown
PVI – povidone iodine, control – saline or no treatment.

Subgroup analysis by PVI application method showed that the decrease in SSI incidence was statistically significant when PVI was administered as irrigation or spray compared to saline or no treatment. However, PVI application as a lavage or scrub did not cause any significant decrease in SSI vs. control (Figure 6). Further, the test for subgroup analysis revealed no statistically significant subgroup effect (p = 0.38), suggesting that the PVI application method does not modify the effect of PVI. However, a smaller number of studies contributed to data in the lavage and scrub subgroups.
Subgroup analysis by surgery category showed inconsistent effects of PVI on SSI incidence. Statistically significant results were seen in abdominal, gynecological, spinal, and orthopedic procedures, whereas no statistically significant effects were seen in accident surgery (Figure 7). The test for subgroup differences was not statistically significant (p = 0.05). The heterogeneity statistic (I 2 value) and the number of studies in each subgroup were inconsistent.
Figure 8
Forest plot for subgroup analysis of type of procedure in studies using a random effects model. Risk ratios and 95 confidence intervals are shown
PVI – povidone iodine, control – saline or no treatment.

Stratification by the type of procedure (elective, urgent, or mixed) showed a reduction in SSI incidence, which was statistically significant and which was consistent across all subgroups (Figure 8). The test for subgroup differences indicated no statistically significant subgroup effect (p = 0.94), suggesting that type of procedure does not modify the effect of PVI. Heterogeneity was low to moderate in the subgroups.
Discussion
The present study provides current and valuable information on the usefulness and efficacy of PVI in decreasing SSI incidence across various surgical categories and procedures. This meta-analysis showed that topical application of PVI in the preoperative or intraoperative phase for the 59 RCTs resulted in a decreased incidence of SSI by 30%. This favorable effect was mainly observed for patients undergoing abdominal and gynecological (cesarean section) surgery with a 22% and 19% reduction in SSI incidence. The heterogeneity values were low to moderate for these surgical categories, providing confidence in the values of the pooled risk ratios. Although beneficial effects were also seen for orthopedic and spinal surgery, the number of studies was insufficient and the I2 heterogeneity statistic high. The high heterogeneity values can be attributed to diverse patient characteristics as some studies were carried out in pediatric populations.
Significant and consistent benefits of PVI were also observed in elective, urgent, and mixed procedures, although the studies showed moderate heterogeneity. This heterogeneity can be attributed to the type of surgery performed and variable patient characteristics. The effects of PVI were not consistent depending upon the application method, although the subgroup effect was not significant. PVI administration as irrigation or spray resulted in a significant decrease in SSI incidence, whereas administration as a lavage or scrub did not provide significant benefit. However, the number of studies for the lavage and scrub groups was too small (n = 10 studies) to allow a definite conclusion to be made.
The type of comparator (saline or no treatment) does not modify the effect of PVI. However, studies carried out using either comparator showed a significant decrease in SSI when PVI was administered in any form compared to the control.
In only one study, Muller et al. [59], laparoscopic surgery was done in 78.3% of procedures in the PVI group and 81% of procedures in the control group. However, individual SSI incidences for the laparoscopic vs. conventional methods were not reported.
Although the current meta-analysis suggests that preoperative or intraoperative use of PVI is associated with an overall decrease in SSI compared to saline or no treatment, it is essential to understand the limitations of the studies included. Risk of bias analysis showed uncertain quality for most domains for the RCTs, indicating the possibility of selection, performance, and detection bias, raising concerns over the reliability of the studies. Adequate methods of allocation concealment and blinding were unclear in the studies, making it challenging to assess the trial quality. Additionally, prophylactic antibiotic administration and post-operative antibiotic use were not consistent between the RCTs. Preoperative antibiotics were administered to PVI and control groups in 28 studies without a relationship between SSI incidence and antibiotic use. Antibiotic use can significantly affect SSI rates and produce an inflated estimate of SSI reduction which may not be related to PVI treatment. Follow-up times for observation of SSI development differed between the studies. The current CDC definition for an SSI recommends a follow-up time of 30 days [8], whereas a few studies reported shorter time frames for post-surgery observation, which is an additional cause of variability for the outcome. Included studies were heterogeneous with regards to populations, prophylactic antibiotic use, and time of PVI application. Since several studies included in the meta-analysis were published before 1990 (n = 27), it is essential to consider possible changes in surgical practices that can modify the benefits of PVI.
Conclusions
Regardless of possible limitations, the present meta-analysis indicates that preoperative and intraoperative use of PVI is beneficial in decreasing SSI incidence. However, for surgeons to justify the use of PVI, its application must be carried out taking into consideration current surgical practices and procedures.
What is the ‘take-home’ message for the clinician?
Antisepsis of the surgery region and antibiotic prophylaxis are critical preoperative preventative treatments. In visceral surgery, intraoperative wound irrigation with povidone-iodine solution decreases SSI.The use of intra-operative PVI may help to lower SSI rates. Because there are few recent studies and surgical techniques may have changed, modern, properly powered, and well-designed clinical trials, stratified by antibiotic treatment and wound contamination, and using an updated and uniform definition of SSI, are needed to validate these findings.
Review criteria: how did you gather, select and analyze the information you considered in your review?
A systematic literature search was conducted of MEDLINE (PubMed) and Cochrane Register of Controlled Trials (CENTRAL) in June 2021. No time limit was applied as several studies were published earlier than 1990. The following search terms were used in various combinations: surgical site infection, wound infection, SSI, post-operative, povidone-iodine, betadine, irrigation, and spray, and lavage, intraoperative and anti-infective agents.