Introduction
Atopic dermatitis (AD) is a chronic, inflammatory dermatosis with periods of exacerbation and remission. The clinical presentation includes dryness of the skin and eczema-like eruptions. Persistent itching of the skin causes sleep disturbances and interferes with daily activity [1–3]. The most frequently mentioned factors responsible for the development of the disease include immunological and genetic disorders, environmental factors, epidermal barrier defects, and disturbed microbiome (Figure 1) [4].
The main immune disorders are characterised by the overexpression of Th2 cytokines (T helper type 2 cells) such as interleukins: IL-4, IL-5, and IL-13, and chemokines CCL17, CCL18, and CCL22 [5]. The interaction of IL-4 and IL-13 with the IL-4R (interleukin 4 receptor) promotes Th2 differentiation and mediates allergic adaptive immune response. They are considered critical cytokines in the pathogenesis of atopic diseases [6]. Due to the recurrent nature, eruption in exposed areas of the skin, and persistent itching, atopic dermatitis significantly reduces patients’ quality of life. So far, treatment options for more severe forms of the disease have been limited. Treatment with immunosuppressive drugs such as systemic corticosteroids, cyclosporin A (CyA), methotrexate (MTX), azathioprine (AZA), and mycophenolate mofetil (MMF) is associated with the possibility of serious side effects, limited effectiveness, duration of treatment, or registration indications. The prospect has changed with the advent of biological drug registrations.
Dupilumab is the first monoclonal antibody approved in the EU and by the FDA. Dupilumab is indicated for the treatment of moderate to severe atopic dermatitis in adults and adolescents of 12 years of age and older and severe atopic dermatitis in children from 6 to 11 years old who are candidates for systemic therapy [7]. That drug is a monoclonal antibody targeting the a subunit of the IL-4 receptor and is responsible for blocking the signalling of IL-4 and IL-13. It is responsible for inhibiting immune disorders characterized by the Th2 phenotype [8, 9]. Clinical trials conducted for over 10 years confirmed the efficacy and safety of dupilumab in the treatment of AD [10–12]. The most common adverse reactions during treatment with dupilumab are mild to moderate such as nasopharyngitis, upper respiratory tract infection, injection site reactions, skin infections, and conjunctivitis [13].
Aim
The study aims to assess the efficacy and safety of dupilumab treatment in patients with moderate and severe atopic dermatitis in everyday practice, and to compare them with the results of clinical trials and experiences from other research centres.
Material and methods
We collected data from September 2019 to January 2021 on patients treated with dupilumab for moderate to severe atopic dermatitis referred to our department. The eligibility criteria for treatment were as follows: age over 16 years, diagnosis of moderate to severe AD, SCORAD (SCORing Atopic Dermatitis) ≥ 25, and no significant abnormalities in basic laboratory tests and chest X-ray. Exclusion criteria included pregnancy or breastfeeding, lack of patient compliance, severe infections, active parasitic infection, and hypersensitivity to the active substance or excipients. No specific time interval was required between other general therapies used by the patients; concomitant treatment with topical corticosteroids or calcineurin inhibitors was also acceptable. We included all treated patients in the department for the analysis. Patients were treated subcutaneously with dupilumab at an initial loading dose of 600 mg followed by 300 mg every other week (in line with the Summary of Product Characteristics). The following data were collected from the medical records: disease picture, age of AD onset, comorbidities, previous treatment, changes in the objective and subjective parameters of the disease, and adverse events. If available, eosinophil counts were also collected. Clinical evaluation was performed at baseline and after 4 and 8 weeks from the beginning of the treatment using validated scores of SCORAD, Pruritus Analogue Scale, and Sleep Disorder Analogue Scale (VAS Pruritus and VAS Sleep Disorder, range: 0–10).
The average age of the patients was 29.9 years, including one person under 18. The group of 10 patients consisted of 6 women and 4 men. The most common comorbidities were allergic diseases (70%). Other illnesses, including mental or psychological disorders (20%), hypertension, and cardiovascular disorders (10%), were less common. Patients’ prior therapies included: oral corticosteroids (60%), followed by cyclosporine (50%), phototherapy (UVB) (10%), and methotrexate (10%). The 2 youngest patients, 17 and 19 years old, received only topical therapy. All 10 patients completed the 8 weeks of treatment with dupilumab. Some patients continued therapy for 18 weeks.
Results
At baseline, the mean SCORAD score was 53.88. After 4 weeks of treatment, the mean SCORAD score was 31.77. After 8 weeks of treatment, the mean SCORAD score was 25.8. The mean percentage reduction of the SCORAD score was 41.04% after 4 weeks and 52.16% after 8 weeks. After 4 weeks of treatment, 50% of patients achieved the SCORAD50; after another 4 weeks, the percentage increased to 60.0%. The mean VAS severity of pruritus was 6.7 at baseline, 4.27 after 4 weeks, and 3.2 after 8 weeks. The mean percent reduction in pruritus was 36.3% at week 4 and 52.2% at week 8 (Figures 2, 3).
Figure 2
Assessment of disease severity in SCORAD scales (B), pruritus severity in VAS (C), sleep disturbances (A) in VAS during 8 weeks of dupilumab treatment in patients with moderate or severe AD
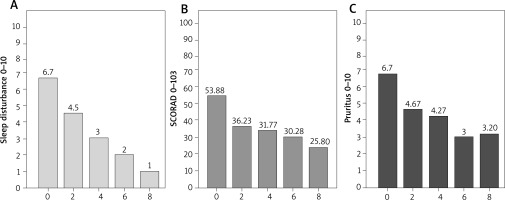
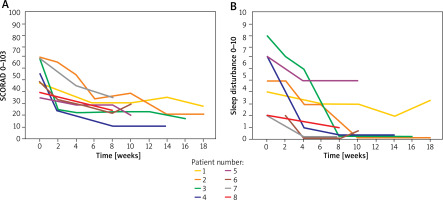
Figure 3
Assessment of disease severity using the SCORAD scales (A), sleep disturbances (B) on the VAS scale during the 8 weeks of dupilumab treatment in individual patients with moderate to severe AD
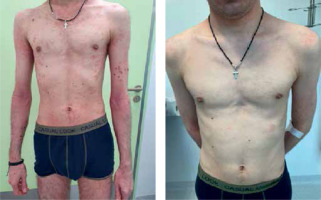
Some patients continued treatment for more than 8 weeks, which was associated with an even better response (Figure 4). Patients with more severe AD at baseline had better treatment outcomes.
Figure 4
Clinical improvement during treatment with dupilumab in a patient with atopic dermatitis (after eight administrations of dupilumab)
Eosinophilia (> 500/mm3) was detected in 60% of the patients at the start of the study and in 70% of the patients at the end of the treatment. In addition, we found no other laboratory abnormalities. Conjunctivitis was the most common adverse reaction observed in 3 patients and persistent facial erythema in two. In our patients, conjunctivitis was most often treated with artificial tears or drops of hyaluronic acid and resolved spontaneously. In 2 cases, treatment with topical steroids and antimicrobials was required.
Discussion
Dupilumab is a fully human IgG4 monoclonal antibody directed against the a subunit of the interleukin 4 receptor. It inhibits signalling the type I receptor (IL-4Rα/γc), preventing binding to IL-4 and through the type II receptor (IL-4Rα/IL-13Rα), preventing both IL-4 and IL-13 signalling. Receptors are located on the surface of cells participating in Th2 responses: B lymphocytes, eosinophils, dendritic cells, monocytes, macrophages, basophils, and keratinocytes [5, 8, 9]. In vitro studies were conducted where transcripts of skin biopsy samples were analysed before and after the treatment of patients suffering from moderate to severe AD who were treated weekly with dupilumab 150 or 300 mg compared to placebo. The expression of genes involved in AD pathogenesis was decreased in the skin biopsy of dupilumab-treated patients, and molecular changes were accompanied to improve accompanied to improve clinical outcomes [14]. Dupilumab significantly reduces serum levels of CCL17 (thymus- and activation-regulated chemokine), which is the crucial regulator of Th2-dependent immunity and a specific biomarker of AD activity [15]. The three main phase 3 studies have confirmed the efficacy and safety of dupilumab in previous trials and extended further elements such as the length of treatment and combination with other conventional therapies [16–18]. The research was conducted as part of the global LIBERTY AD program which covered over 2,500 patients. Dupilumab monotherapy (SOLO 1 and SOLO 2) and dupilumab therapy co-administered with topical corticosteroids (CHRONOS) were evaluated during the studies. The studies included adult patients with moderate to severe AD whose topical treatments were not sufficient. In SOLO 1, corresponding improvement appeared in 85 (38%) patients who received dupilumab every other week and 83 (37%) who received dupilumab weekly, compared with 23 (10%) who received placebo. Similarly, in SOLO 2, the primary endpoint was in 84 (36%) patients who received dupilumab every other week and 87 (36%) who received dupilumab weekly, compared with 20 (8%) who received placebo [19–22]. Treatment with dupilumab also showed a rapid and sustained reduction in pruritus and improved quality of life from the first dose in the indicated studies. The response to treatment gradually increased and was sustained until the end of treatment [12].
Due to the proven effectiveness and high safety profile, clinical trials were conducted in lower age groups. Two hundred and fifty teenagers were involved in LIBERTY AD ADOL. The study participants were from 12 to 17 years of age (the mean of 14 years), and the mean duration of the disease was 12 years. More than 40% of the respondents had previously received systemic therapy, most often steroid therapy. In the group of patients receiving dupilumab every 2 weeks, 80.5% experienced a significant clinical improvement at week 16 (compared to placebo, 23.5%) [23]. In a 16-week placebo-controlled phase III study in children ≥ 6 to < 12 years of age with severe atopic dermatitis (AD), dupilumab significantly reduced the signs and symptoms of the disease with acceptable safety [24, 25]. Similar results were observed in children with severe AD in a lower age group, from 6 to 11 years. The combination of dupilumab with topical corticosteroids was more effective than a placebo. Response to treatment was related to weight: the optimal dose of dupilumab for children under 30 kg was 300 mg every 4 weeks, while for children weighing 30 kg or more, the optimal dose was 200 mg every 2 weeks. LIBERTY AD PRESCHOOL is evaluating the safety and efficacy of a single dose of dupilumab in children between 6 months and 6 years. The first reports are promising [26].
The most common side effects of dupilumab in patients with atopic dermatitis include injection site reactions, conjunctivitis, headache, and upper respiratory tract infections [13, 27]. A systematic review and meta-analysis of dupilumab efficacy and risk of adverse events included 22 unique studies involving 3,303 patients with atopic dermatitis. Conjunctivitis was the most common adverse event, reported in 26.1% of patients. Adverse events, such as alopecia areata, arthritis, and flushing, were less commonly reported. The effect of dupilumab on the course of alopecia areata is inconclusive. The clinicians observe face erythema after treatment with dupilumab, but the mechanism is unknown [13]. Another review showed that patients treated with dupilumab had a higher risk of injection site reactions, headache, conjunctivitis, and a lower risk of skin infection and exacerbation than patients treated with placebo [27]. Conjunctivitis associated with dupilumab therapy is most commonly bilateral, mild to moderate in intensity. Patients reported itching, burning, tearing, and foreign body sensations. Most of the reported cases of conjunctivitis either spontaneously remitted or resolved after the initiation of treatment [28–30].
After the drug registration, data from the TREAT NL registry (Treatment of Atopic eczema, the Netherlands) on the long-term use of dupilumab (up to 7 years) in patients with AD were published. The registry included 221 patients who started dupilumab treatment in everyday clinical practice. After 84 weeks of treatment, there was an average decrease in the Eczema Area and Severity Index by 15.2 and the Dermatology Life Quality Index (Dermatology Life Quality Index) by 17.2. Serious adverse events and events requiring urgent treatment occurred in 69 patients. The most common adverse actions were in the eyes (46/225) – mainly keratitis and conjunctivitis. Mild side effects were not recorded in the study. Fourteen patients discontinued treatment, out of which seven did not experience any effects of the drug [31].
In the available studies, there were no clinically significant changes in routine laboratory parameters such as morphology, biochemistry, or urine testing during treatment with dupilumab. This study supports the use of dupilumab in the systemic treatment of moderate to severe AD that does not require laboratory monitoring [32]. In addition, dupilumab significantly reduced AD symptoms, including itching, sleep, pain, anxiety, and depression in adults with moderate to severe AD [33]. Our analysis supports the efficacy and safety of dupilumab for treating moderate to severe atopic dermatitis. It rapidly reduces signs and symptoms, pruritus, and sleep disturbance, and improves patients’ overall quality of life. This corresponds to the experience and results obtained by other researchers [34–37]. Real-world data show that dupilumab is an effective and well-tolerated treatment of atopic dermatitis. The data we present have limitations such as a small number of patients, a short follow-up period, and a retrospective evaluation. The use of dupilumab does not cause serious side effects and provides clinical improvement in patients with moderate to severe AD.
Conclusions
Our experience and observation showed a significant improvement in the course of AD as measured by the SCORAD scale, a reduction in objective symptoms such as pruritus and sleep disturbances between the starting point and the fourth week of treatment, with a further significant decrease in the eighth week of therapy. The efficacy and safety of dupilumab treatment in real life were consistent with the previously known results of clinical trials.