Introduction
The first FDA-approved bioresorbable scaffold (BRS) Absorb BVS (Abbott Vascular, USA) demonstrated a higher rate of scaffold thrombosis when compared to current-generation metallic drug-eluting stents (DES) [1]. It is believed that the bulky strut thickness of 156 µm, exceeding nearly twice that of contemporary metallic DES, is responsible for the higher thrombotic potential of this technology [2].
The speed and quality of stent/scaffold strut coverage by tissue are strongly impacted by strut thickness and shape, which influence shear stress and blood flow dynamics, affecting platelet accumulation and endothelial cell growth [3–5]. Regardless of strut height, non-streamlined scaffold struts influence blood flow recirculation and low flow shear rates and prolong particulate residence time [6]. Low endothelial shear stress influences endothelialization rates, and taller strut height induces an environment of procoagulant and proinflammatory elements, which can lead to a greater quantity of thrombus and also accelerate turnover of endothelial cells, making reendothelialization more difficult [7, 8]. Preclinical studies demonstrated that strut thickness influences thrombogenicity, with struts that are < 100 µm having smaller thrombi than struts that are > 100 µm [9]. Therefore, the reduction in strut thickness resulting in improved vascular healing with neointimal strut coverage is a crucial concept in developing new BRS.
In this study we aimed to evaluate the short-term healing response using optical coherence tomography (OCT) following implantation of a new-generation sirolimus-eluting amorphous PLLA-based BRS (Amaranth Medical, USA) with ultra-thin strut (98-µm, AMA-98) and thin strut (115-µm, AMA-115) thickness in porcine coronary arteries.
Material and methods
Device description
Scaffolds used in this study have almost identical ring design and different strut thicknesses: the ultra-thin strut AMA-98, with strut thickness of 98 µm; and the thin strut AMA-115, with strut thickness of 115 µm. The only difference between the 2 versions is a slightly increased surface area coverage at rated burst pressure in AMA-98 when compared to AMA-115 (respectively: 22% vs. 21% for 3.0 mm scaffolds). Both scaffolds were manufactured by the same company (Amaranth Medical, USA) using an ultra-high molecular weight bioresorbable polylactide-based polymer. Devices are coated with a matrix consisting of a 1 : 1 polymer : drug ratio of sirolimus plus Poly D-Lactide polymer and with a sirolimus dose density of ~96 µg/cm2. The core AMA-BRS technology was described in detail previously [10–12]. Both devices underwent extensive preclinical testing and are currently evaluated in the first-in-men clinical trials.
Study design
The Institutional Animal Care and Use Committee approved all studies, and all animals received care in accordance with the Guide to Care and Use of Laboratory Animals. All animals underwent endotracheal intubation and were maintained on continuous inhalation of 1–3% isoflurane. Anticoagulation with heparin was achieved during the procedure (500–5,000 U) to maintain an activated clotting time (ACT) ≥ 250 s. In this study either AMA-95 (n = 12, size 2.5, 3.0, 3.5 × 18 mm) or AMA-115 (n = 15, size 2.5, 3.0, 3.5 × 18 mm) were implanted targeting a stent-to-artery ratio of 1.1 : 1 under intravascular ultrasound guidance in 26 porcine coronary arteries of 16 Yucatan mini swine. In vivo structural integrity and recoil, as well as strut-vessel wall interactions, were evaluated longitudinally at 14 and 28 days using optical coherence tomography.
Optical coherence tomography imaging
OCT images were recorded at post-implantation day 0 and at follow-ups using the ILUMIEN PCI Optimization System (St. Jude Medical, USA) following a previously published protocol [8] and the cross-sectional morphometry parameters were measured with commercial software (ILUMIEN OPTIS, St. Jude Medical, USA) as previously described [5–7] at 2-mm axial intervals. The following cross-sectional morphometry parameters were measured and calculated as previous described [10]: lumen area, inner and outer scaffold area, percentage area of stenosis. Absolute late recoil was measured as the mean scaffold area at baseline (post-implant) minus the mean scaffold area at follow-up. Relative late recoil (normalized to scaffold size) was calculated as: [(absolute late recoil)/baseline mean scaffold area] × 100 [11].
In order to determine the presence of strut tissue coverage in AMA-BRS, the thickness of the endoluminal bright border in 300 frames from 10 scaffolds struts located close to the 12 o’clock position was measured. The threshold for coverage was 30 µm, which corresponds to the average interobserver measurement of the endoluminal bright border of the strut [12]. Evaluated struts were assigned to 1 of the 3 following categories: covered with complete inter-strut neointima (embedded), covered without complete inter-strut neointima (protruding covered) and struts without evidence of coverage (uncovered).
Statistical analysis
Statistical analysis were performed using SAS statistical software (version 9.4; SAS Institute Inc. NC). Continuous variables were expressed as mean ± SD with the median and interquartile range used for variables with non-normal distributions. A mixed model compared differences between the 2 treatments (AMA-98 vs. AMA-115) while accounting for dependent observation over time. Furthermore, this model contained a random effect with compound symmetric covariance structure to account for multiple scaffolds implanted in the same pig. The device, time, and interaction between time and device were modeled as fixed effects. Scheffe’s post hoc test was applied to compare differences between time points and differences between treatments at each time point. A nonparametric test was used for those dependent variables with non-normal distributions to account for multiple scaffolds implanted in the same pig. All tests were 2-tailed with a type I error held at 0.05.
Results and discussion
On day 0, post-implant OCT indicated that all scaffold struts were fully apposed to the vessel wall with no strut malapposition observed in any of the implanted vessels. No post-dilation was performed. In total, 536 cross-sections and 4223 struts were evaluated to sequentially assess In vivo structural integrity and recoil, as well as strut-vessel wall interactions and short-term strut healing response at 14 and 28 days (Figure 1). The percentage of embedded struts was significantly higher at 14 days in the AMA-98 group (AMA-98 = 97.7% (95.2, 89.1) vs. AMA-115 = 71.0% (69.5, 92.2), p = 0.007). Conversely, the presence of protruding covered struts was more commonly seen in AMA-115 at 14 days (AMA-98 = 1.7% (1.7, 4.8) vs. AMA-115 = 17.3% (7.6, 17.3), p = 0.012). There was no difference in the uncovered struts between AMA-98 and AMA-115 at 14 days. At the 28-day follow-up no statistical difference in coverage was observed between tested devices. There was no difference in the reference lumen area between AMA-98 and AMA-115 groups. However, abluminal scaffold area was smaller immediately after implantation and during 14 days of follow-up in the AMA-98 group compared to AMA-115. Percent area stenosis was similar in both scaffolds at 14- and 28-day follow-up. The results of OCT morphometric analysis are summarized in Table I.
Figure 1
Comparison of serial OCT variables at 14 and 28 days follow-up between AMA-98 and AMA-115. There was a higher percentage of embedded struts at 14 days in the AMA-98 group, while more protruding covered struts were observed in the AMA-115 group at 14 days. Values are expressed as median (25th–75th percentile)
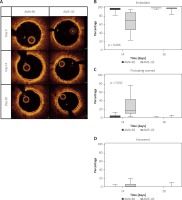
Table I
Morphometric OCT data
| Parameter | 14 days | 28 days | P-value* |
|---|---|---|---|
| Lumen area [mm2]: | |||
| AMA-95 | 6.12 ±0.63 | 5.12 ±0.62# | 0.001 |
| AMA-115 | 6.97 ±0.90 | 6.14 ±0.95# | |
| P-value† | 0.007 | 0.076 | |
| Endoluminal scaffold area [mm2]: | |||
| AMA-95 | 6.78 ±0.58 | 6.42 ±0.51 | 0.102 |
| AMA-115 | 7.64 ±0.96 | 7.47 ±0.99 | |
| P-value† | 0.007 | 0.120 | |
| Abluminal scaffold area [mm2]: | |||
| AMA-95 | 7.73 ±0.63 | 7.32 ±0.56# | 0.098 |
| AMA-115 | 8.71 ±1.00 | 7.99 ±1.44# | |
| P-value† | 0.005 | 0.353 | |
| Area stenosis (%): | |||
| AMA-95 | 9.87 ±2.80 | 20.4 ±4.8# | < 0.001 |
| AMA-115 | 8.78 ±1.66 | 17.96 ±6.43# | |
| P-value† | 0.547 | 0.436 | |
| Absolute scaffold recoil [mm2]: | |||
| AMA-95 | –0.35 (–0.57, –0.10) | 0.02 (–0.13, 0.28)# | < 0.001 |
| AMA-115 | –0.10 (–0.43, 0.12) | 0.07 (–0.07, 0.32)# | |
| P-value† | 0.335 | 0.795 | |
| Percent scaffold recoil (%): | |||
| AMA-95 | –5.54 (–9.32, –1.85) | 0.22 (–2.11, 4.20) | < 0.001 |
| AMA-115 | 1.46 (–6.11, 1.73) | 1.02 (–0.90, 4.49)# | |
| P-value† | 0.118 | 0.872 | |
The impact of strut thickness on early vascular healing and neointimal proliferation has been well described. Studies of coronary flow dynamics suggest that strut thickness induces laminar flow disturbances around the struts, potentially increasing thrombogenicity of the device [8]. Early BRSs have achieved an acute biomechanical performance comparable to metallic stents but at the expense of greater strut thickness. Novel PLLA formulations promise to improve biomechanical properties of current generation BRS devices. Our previous studies demonstrated higher overexpansion capabilities and dramatic improvement in resistance to fracture under static and dynamic conditions with ultrahigh molecular weight amorphous polymers [10, 12, 13].
The objective of this study was to evaluate the impact of strut thickness on early vascular healing using OCT over the first month after implant, when the scaffold interactions with blood flow matter more than the artery wall’s response to injury. Our results demonstrated that AMA-98 displayed superior strut coverage in the early phase of vessel healing compared to AMA-115. At 14 days the percentage of embedded struts was significantly higher in the AMA-98 group. Conversely the rate of protruding covered struts at 14 days was greater in the AMA-115 group. Interestingly, the difference occurred despite the fairly modest difference in strut thickness, corroborating earlier computer modeling and in vivo research on this topic [9]. There was no difference in the uncovered struts rate between the two tested scaffolds at any timepoint. Also, the more favorable strut coverage with thinner struts did not occur at the expense of higher late recoil, which was equivalent in both devices at 1 month. As expected, the neointimal proliferation expressed by percent area stenosis was low and similar in both groups.
The present study has some limitations that are important to discuss. The study was performed in healthy coronary arteries in a swine model. All scaffolds were implanted in the main coronary artery segments, avoiding large side branches (> 2.0 mm), and the number of implants at each time point was too small to draw any definite conclusion. Our findings cannot predict their clinical performance among patients with atherosclerotic burden.
Conclusions
Our data demonstrate that the ultra-thin strut AMA-98 featured more favorable strut coverage characteristics at early follow-up in normal porcine coronary arteries compared to the thin strut AMA-115 with similar ring design, at no expense to acute or late recoil. Interestingly, the semiquantitative differences were significant even though the strut thickness between the 2 devices differed only by 17 µm.








