Introduction
Lateral local recurrence (LLR) has become the major cause of local recurrence (LR) for advanced low rectal cancer since the full adoption of total mesorectal excision (TME) has dramatically reduced the LR rate at the anastomosis. Nowadays, there is increasing attention focused on lateral local control, especially for those showing an enlarged lateral lymph node on the primary evaluation magnetic resonance imaging (MRI) scan [1]. Moreover, neoadjuvant chemoradiotherapy (nCRT) + TME has been proved not sufficient in eradicating LLR in patients with pretreatment suspected lateral lymph node metastasis (LLNM) [2], and adding lateral lymph node dissection (LLND) to these patients can reduce the recurrence rate from 19.5% to 5.7% [3]. Meanwhile, even though the noninferiority of ME alone to ME + LLND was not confirmed, in the real world, there has been a sharp decrease in the prophylactic LLND rate even in Japan in order to avoid overtreatment [4]. Harmonizing the evidence for decades, more and more centers around the world tend to adopt nCRT + TME + LLND as the optimal strategy for patients with clinical lateral metastasis, especially in Asian countries.
LLND is a complex and challenging procedure, not only because of the anatomical complexity of the lateral pelvic compartment but also its close relation to the urinary and sexual dysfunction postoperatively. Based on the findings of several studies, the inferior vesical artery (IVA) branch from the internal iliac artery (IIA), around which is the most frequent site of LLNM, the combined IVA resection is easier to perform and often required in securing an en bloc dissection of metastatic lymph nodes surrounding the inferior vesical vessels (IVVs), also named the no. 263D lymph nodes according to the Japanese classification. However, there are concerns that LLND with IVV resection would lead to an increasing rate of urinary dysfunction [5, 6]. It is assumed that bilateral IVV resection would more likely to lead to urinary dysfunction. Recent studies showed that there was no evidence of impaired urinary function if autonomic nerve-preserving surgery was performed [7, 8]. Postoperative voiding function has a close relation to autonomic nerve injury, urinary retention or constant need of self-catheterization, and indwelling catheterization could dramatically lower the patients’ early-stage postoperative quality of life (QOL). Whether IVV resection causes autonomic nerve damage and whether it can be performed with autonomic nerve preservation are questions that have not yet been fully answered.
Since 2016, our team has performed LLND routinely with IVV resection following a fascial space priority approach (FSPA), which was introduced with a video in our prior article [9], characterized by prioritized autonomic nerve preservation and IVV resection.
Aim
This study aimed to compare the postoperative early-stage voiding function between patients who underwent uni- versus bilateral IVV resection and to elaborate on the detailed procedure of intraoperative autonomic nerve preservation during IVV resection with an attached video.
Material and methods
Patient selection and data collection
Between May 2017 and October 2022, a total of 106 patients with low rectal cancer underwent therapeutic LLND at the Department of Colorectal Surgery, Tianjin Union Medical Center (TUMC). Inclusion criteria: lateral lymph nodes with a short axis diameter ≥ 7 mm and < 7 mm but showing malignant signs such as irregular margins and enhancement on pretreatment MRI. Additionally, 10 cases whose primary MRI report indicated N0 but suspected lateral lymph nodes that were found during pre-surgery multi-disciplinary team (MDT) evaluation were also included. The exclusion criteria were patients with preoperative urinary dysfunction, those who underwent combined resection of the urinary organs (except the seminal vesicle) or autonomic nerves, and the presence of distant metastasis. The medical records, the surgical videos and the follow-up data of the patients were reviewed retrospectively. The eighth TNM classification system according to the American Joint Committee on Cancer (AJCC) was used to classify the tumor stage.
According to the clinical guidelines at the Department of Colorectal Surgery, TUMC, the catheter would be removed on the 7th postoperative day (POD) or a day before discharge. The residual urine would be assessed the following morning; residual urine ≥ 100 ml and anytime when acute urinary retention occurs are indicators of re-catheterization; the catheter would be successfully removed until residual urine ≥ 100 ml under ultrasound assessment. Thus, early-stage voiding function could be assessed by the proportion of cases needing re-catheterization and duration (days) from surgery to successful removal of the catheter, and it was used as a primary indicator of urinary function recovery.
Informed consent was obtained from all patients and this retrospective observational study was approved by the Ethics Committee of TUMC.
Surgical procedure
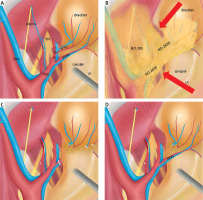
All surgical procedures were performed by laparoscopy; simultaneous LLND was performed following the TME procedure. The fascial space priority approach was routinely adopted for all LLND procedures. The main principle was giving priority to the protection of the ureterohypogastric nerve fascia (UHGNF). The detailed procedure was introduced with a video in our prior article and a new video with animation and narrative was attached here, aiming to emphasize autonomic nerve sparing during IVV resection. All videos included in this study were reviewed. The IVVs were resected as a routine procedure. For patients who underwent unilateral lymph node dissection (LND), the surgical side IVVs and superior vesical vessels (SVVs) were resected, while for patients who underwent bilateral LND, IVVs on both sides were resected; meanwhile at least one side of SVVs was reserved to maintain blood flow to the bladder (Figure 1). The autonomic nerve preservation combined with IVV resection was achieved following a stepwise procedure: firstly, the UHGNF, which contains the ureter, the hypogastric nerve, nerves S2-S4, and the pelvic plexus, was dissected and protected; secondly, the vesicohypogastric fascia (VHGF) was dissected along the side wall of the bladder; thus the inner border of the no. 263D lymph nodes was defined, and the IVVs were resected where they run through the junction of the UHGNF and the bladder (the UHGNF-bladder plane) before they branch to the bladder wall (Figure 1). It is common that there are more than 2 pairs of IVVs (IVA and inferior vesical vein) on each side, at the intersection of the IVVs and the VHGF-bladder plane. Care should be taken not to injure the S4 and the pudendal nerve. In some cases, the nerves are recognizable (see the Video  ), and small lymph nodes located at the UHGNF-bladder junction could easily be neglected.
), and small lymph nodes located at the UHGNF-bladder junction could easily be neglected.
Figure 1
Diagram of the anatomy of the left lateral compartment and the surgical procedure. A – anatomy of the left lateral pelvic compartment; B – lateral lymph node classification according to Japanese classification; red arrows show the prior dissection of the UHGNF – bladder plane; C – surgical field after unilateral LND; D – surgical field after bilateral LND
IVVs – inferior vesical vessels, Ur – ureter, SVVs – superior vesical vessels, UHGNF – ureterohypogastric nerve fascia, IPA – internal pudendal artery, EIVs – external iliac vessels, IIVs – internal iliac vessels, ON/Ovs – obturator nerve and obturator vessels, SN – sciatic nerve.
Results
Patients’ characteristics
The total of 106 patients who underwent LLND were divided into 2 groups: 75 in the unilateral LND group and 31 in the bilateral LND group. The clinical characteristics were compared between the two groups (Table I). There were no significant differences between the groups with regard to age, gender, BMI, short-axis of maximum lateral lymph node (LLN) on MRI, preoperative neoadjuvant therapy rate, type of surgery, cT stage, cN stage and overall hospital stay duration. Of note, 10 cases were primarily reported as N0 by the radiologist and with suspected lateral lymph nodes identified during pre-surgery MDT, 4/10 with pathologically positive LLN, 1/10 with bilateral LLN.
Table I
Clinical characteristics of patients who underwent unilateral and bilateral LND
Comparison of perioperative data between groups
All operations were performed laparoscopically with autonomic nerve preservation following a fascial space priority approach and IVV resection was performed as a standard procedure during LLND. Stepwise surgery is illustrated in the attached video. No case was converted into laparotomy. The perioperative outcomes of the patients who underwent unilateral LND and bilateral LND are summarized in Table II. There were no significant differences between groups with regard to estimated blood loss, surgical procedure, combined organ resection, complication rate, histological type, margin status, post-operative T stage and re-catheterization rate. Bilateral LND required a significantly longer surgical time (380 min vs. 500 min, p < 0.01) but with similar overall blood loss (p = 0.06). Patients who underwent bilateral LND had more lymph nodes harvested than people who underwent unilateral LND (8 vs. 14, p = 0.001). A higher proportion of patients with pathologically positive lateral pelvic lymph node (LPLN) was identified in the bilateral LND group (87.1% vs. 64.0%, p = 0.017). A higher vascular invasion rate (p = 0.042) and higher post-operative N stage (p = 0.022) were found in the bilateral LND group. No deaths were observed in either group.
Table II
Operative and post-operative characteristics compared between patients who underwent unilateral and bilateral LND
Risk factors for overall catheterization duration (days)
The clinical risk factors of the patients with prolonged catheterization duration (days) are shown in Table III. Gender, age, type of LLND, combined organ resection, neoadjuvant therapy, sphincter preserving or not were not significantly associated with longer catheterization duration. Grade 3 complications were significantly associated with an increase of days of catheterization (p = 0.039). Additionally, patients who underwent only LLND had a significantly shorter duration of catheterization compared to those who underwent simultaneous TME + LLND (p = 0.013).
Table III
Factors influencing overall catheterization duration (days)
[i] Complication with CD grade ≥ III included ileus, urinary infection, urinary fistula, intra-abdominal infection, deep-vein thrombosis in the lower extremity requiring placement of an inferior vena cava filter. LND – lymph node dissection, LLND – lateral lymph node dissection, CD – Clavien-Dindo, TME – total mesorectal excision.
Discussion
LLND has gained increasingly attention since recent research found that nCRT + TME might be insufficient for advanced low rectal cancer with suspected LLNM [10]. Meanwhile, in patients with suspected LLNM, LLND showed benefits in decreasing the LLR rate, yet its benefit in improving OS has not been confirmed [11, 12]. It is advisable for colorectal surgeons to be proficient in LLND, as it may be beneficial in selected cases. Nowadays, LLND is still not routinely performed because of the complexity of the surgical procedure and concerns about postoperative urinary and sexual dysfunction.
To simplify the surgery, IVV resection was considered promising [13]. The tricky point during LLND is to deal with the IVVs. Combined IVV resection is a simpler procedure than IVV preservation. Moreover, IVV resection is sometimes necessary to ensure a curative resection in cases with enlarged lymph nodes attaching or surrounding the main trunk of the IVVs and when multiple lateral lymph node metastases are suspected. Based on these considerations, we have been routinely performing IVV resection during LLND since 2016. In the video we have attached here, we elaborate on the detailed procedure of IVV resection with autonomic nerve sparing, using the fascial space priority approach. Generally, a plane constituted by the bladder and the UHGNF (the ureter and the autonomic nerve plexus) was isolated first; we call it the UHGNF-bladder plane. The IVVs were resected at the intersection where the IVVs join with the plane. All cases were performed following the stepwise procedure. Videos were recorded for every surgery. No major intraoperative complications were identified.
Studies have shown that mesorectal excision with LLND was associated with a higher rate of urinary dysfunction in comparation with mesorectal excision only [14–16]. Compared to procedures without autonomic nerve preservation, LLND with an autonomic nerve-sparing technique may help protect against and reduce the incidence of postoperative urinary dysfunction [17, 18]. Manabe et al. [5] found that at discharge, the incidence of postoperative urinary dysfunction significantly increased with bilateral IVA resection compared to unilateral resection, with rates of 8.7% (2/23) for unilateral and 77.8% (7/9) for bilateral resection. Similarly, Sadakari et al. [19] found that the incidence of urinary retention on the seventh day after surgery (TME + LLND) was 47.1% (9/19) among patients who underwent unilateral IVA resection, and increased to 88.9% (8/9) in those who underwent bilateral IVA resection. There was a significant difference in the incidence of urinary retention between the unilateral and bilateral groups. They also reported that 44.4% (4/9) of patients who received bilateral IVA resection experienced a catheterization duration of up to 4 weeks and 33.3% (3/9) of the patients need long-term (more than 1 year) self-catheterization. In our study the rates of re-catheterization were 29.0% (9/31) in the bilateral IVV resection group and 34.7% (26/75) in the unilateral IVV resection group (Table II), with no significant difference between the unilateral and bilateral groups. Meanwhile, all the catheters were removed successfully (with residual volume less than 100 ml) within 30 days, suggesting that the surgical techniques of IVV resection with UHGNF-bladder plane preservation may be efficient in reducing the risk of re-catheterization associated with bilateral IVV resection.
Urinary catheter placement is a routine process before TME. Postoperative early-stage voiding function is a direct indication of urinary function recovery and is closely related to the short-term quality of life. The optimal time for removing the catheter remains unclear. There is still controversy over whether early removal of the catheter would be beneficial for patients’ urinary function recovery. Some studies highly recommend that the urinary catheter should be removed on the 1st POD to meet the requirements of Enhanced Recovery After Surgery (ERAS), while others suggested that early withdrawal of the catheter might be an independent risk factor for acute urinary retention (AUR), especially for patients with low rectal cancer and LLND [19–22]. Still, other research found that the catheter removed on the 3rd POD had a satisfactory AUR rate and recommended delaying removal of the urinary catheter until the 5th POD for patients who have undergone TME [22, 23]. In this study, the mean distance of the tumors to the anal verge was 4.44 ±2.09 cm in the unilateral LLND group and 4.91 ±3.00 cm in the bilateral LLND group. Traditionally, the catheterization duration is 7 days or until 1 day before discharge in our institution. In the aforementioned articles, self-catheterization and medication treatment were performed for cases with AUR; no further record was available for duration of self-catheterization. Because self-catheterization was generally not acceptable in our country based on the cognitive and ethnic background, indwelling re-catheterization was performed for all cases with AUR or abnormal residual volume included in this study, and overall catheterization duration was recorded until the day of successful removal of the catheter (with residual volume < 100 ml the following morning). No significant difference was confirmed in terms of overall catheterization duration between the two groups (p = 0.336). Postoperative complication was a reason for prolonged catheterization (p = 0.039). In line with other research [13], for the 31 patients who underwent bilateral IVV resection in our study, none suffered postoperative acute ischemic cystitis, suggesting that the SVVs might provide sufficient blood flow to the bladder. Apart from the SVVs, the dorsal vascular complex (DVC) might also play a critical role in maintaining blood flow to the urinary tract.
For decades, different approaches were employed to manage LLNM in the East and West. Prophylactic LLND used to be popular in Japan, while nCRT + TME was the standard of care in the West [24]. However, with the accumulation of new evidence and improvement of preoperative imaging, in the real world, there has been a decline in prophylactic LLND [4] in the East and a growing frequency of LLND in the Western world, especially for cases with enlarged lateral lymph nodes unresponsive to nCRT [25]. In line with the world literature, we adopt the short-axis diameter of lateral lymph nodes ≥ 7 mm before nCRT along with morphological changes as criteria for suspected LLNM [26]. However, among 10 cases in this series whose primary MRI reports showed negative LLN while the surgical team members found suspected lymph nodes during pre-treatment discussion imaging study, 4/10 turned out to be pathologically positive, so we recommend that surgeons should go through the images of every case before surgery. In our study, among the 75 patients who underwent unilateral LLND, the maximum lymph node short-axis was 0.92 cm (range: 0.50–4.92 cm), with 48 of these cases (64%) demonstrating pathological LLNM. In the bilateral LLND group of 31 patients, the maximum lymph node short-axis was 1.20 cm (range: 0.50–2.28 cm), with 27 (87.1%) cases showing pathological LLNM.
Several limitations of this study can be noted. Firstly, due to its retrospective nature, associated bias cannot be excluded, even though the data were collected prospectively in an institution-owned database. Secondly, patients who underwent TME only were not included in this database, and the functional outcome was not compared with patients without IVV resection. Meanwhile, questionnaires for urinary and sexual function were not provided in this study, because the aim of this study was to compare the immediate influence of IVV resection on early-stage voiding function. Further studies are needed to assess the long-term functional outcome.









