Introduction
Echinococcosis, somewhat forgotten in recent years, is considered one of the most dangerous zoonoses in the world, and the tapeworm that causes it is one of the two most dangerous parasites to humans globally. Untreated cases may be associated with mortality rates as high as 90% [1-4].
The present paper is a brief, up-to-date review of the literature on the issue of this disease in five key areas: the epidemiology, routes of infection, clinical symptoms, diagnostics and therapeutic methods. It contains the current guidelines and criteria for patient assessment and eligibility for treatment, which are also presented in the form of practical tables. It also presents data from the Chair and Department of General and Transplant Surgery of the Infant Jesus Hospital, University Clinical Centre of the Medical University of Warsaw, obtained over the past years regarding the treatment of patients with liver echinococcosis.
The overview describes the distribution of patients by age, sex, area of residence and serology results. It includes the trend in the number of patients presenting to the clinic with a diagnosis over the period 2019-2023. This paper presents the contribution of the surgical methods used at the center and the results of post-operative molecular studies. It reports also the complications that occurred and the effectiveness of the treatment.
Epidemiology
Echinococcus species are found on every continent except Antarctica. The majority of cases of echinococcosis occur in China [1, 5].
Echinococcus granulosus and Echinococcus multilocularis, being the respective causes of cystic echinococcosis (unilocular), constituting 73% of cases, and alveolar (multilocular) echinococcosis, also called alveococcosis, constituting 27% of cases, are the two main species of interest from the viewpoint of human infections [2-4]. In 2020, a total of 529 confirmed cases of echinococcosis were reported in the European Union. Echinococcus granulosus was confirmed in 243 of the cases, while E. multilocularis was confirmed in 114. In the remaining 172 cases, the species was not recognized [3].
Cystic echinococcosis is global in scope, while hepatic alveolar echinococcosis is an endemic disease (occurring in the northern hemisphere, including North America, several Asian and European countries such as France, Germany, and Austria). Occasionally, it also occurs in Tunisia and Morocco [5, 6].
In endemic areas, the annual incidence of E. multilocularis infection varies from 0.03 to 1.2/100,000 inhabitants [3, 5-7]. The mean annual incidence of E. multilocularis infection in Europe is 0.64/100,000 people, and in the EU member states it is 0.50/100,000 people [3, 7]. According to official World Health Organization (WHO) data, the most affected areas include Italy, Spain and Eastern Europe [3, 5, 8].
Research conducted in Africa revealed active transmission of E. granulosus between humans and animals (including wildlife) in countries that had previously not been considered endemic, i.e., Libya, Tunisia, Algeria and Morocco. Wells, which are water points shared by animals and people, are considered to be a source of infestation in the dry areas of the countries [9].
In Western Europe, the majority of infected individuals develop symptoms between the ages of 50 and 60, and pediatric cases are reported very rarely. No correlation of infection with the sex of patients was observed, unlike in Asian countries, where infections are diagnosed at the age of 40-50, with a higher percentage of cases being reported in young adults and women [6].
An increasing number of reports are available as regards echinococcosis in people with an impaired immune response.
It was shown to occur in 1% of solid organ transplant recipients, 0.2% of HIV-infected patients, 4.7% of patients with chronic inflammatory or autoimmune diseases, and 8.3% of patients with malignancies. It was suggested that patients living in endemic areas should undergo serological testing prior to initiating long-term immunosuppressive therapy [10].
In the last decade, Poland has been at the forefront of European countries in terms of the number of cases. On average, over the past 10 years, 435 cases of echinococcosis have been reported in people in our country. This amounts to approximately 40 cases per year [1]. The authors of the Annual Epidemiological Report on echinococcosis in Europe provided slightly different data concerning Poland. In 2019, 70 cases of infections were confirmed, and only 18 in the following year [3]. Most cases occurred in the Mazovian, Podlasie, Warmian-Masurian, Lublin, Subcarpathian and Pomeranian provinces [1].
In Switzerland, a country where reliable medical records date back to the 1950s, the annual occurrence of echinococcosis in humans nearly doubled at the beginning of the 21st century [6]. According to the authors, the increase in the number of cases in humans was probably caused by an increase in the fox population after a successful anti-rabies campaign and an increasing number of foxes inhabiting urban areas [1]. By comparison, the fox population in Poland increased from 67,000 individuals in 1995 to 220,000 in 2006 [11].
Numerous authors have emphasized the possible underestimation of epidemiological data, which included only diagnosed cases. It was estimated that the probable incidence might be three times higher than that described in the reports of individual countries [6, 12]. It may be affected by numerous factors. In our observation, despite the obligation to report infectious diseases, national health care systems often fail to keep accurate records and the collective term echinococcosis is used, without distinguishing the clinical form (uni- or multilocular). Moreover, the parasite is particularly prevalent in rural areas. A large proportion of patients never see a doctor, and population-based screening campaigns are extremely rare. The disease in humans is chronic and its course is asymptomatic for a long time. Therefore, a large number of undiagnosed cases may be due to limited knowledge and limited availability of advanced diagnostic techniques in the countries where the parasite mainly occurs [3-5].
Route of transmission
Echinococcus granulosus inhabits the small intestine of the definitive host (dogs). Subsequently, mature proglottids are excreted with feces and disintegrate in the external environment, resulting in egg release. The eggs are extremely resistant to environmental conditions, characterized by high survival rates, and able to remain invasive for over a year. Humans are entirely accidental hosts. They are “a dead end” of intermediate hosts (pigs, sheep, cattle) and become infected by eating products contaminated with animal feces [1, 4].
As regards E. multilocularis, the fox is the main and definitive host. The human is also an accidental host, and the infection most often occurs through the consumption of forest fruits contaminated with fox feces [12, 13]. Research conducted in the years 2009-2013 involving examination of the postmortem tissue of 1546 foxes throughout Poland revealed that 50% of the specimens were infected with this parasite. Soil tests in the Warmian-Masurian Province showed the presence of tapeworm DNA in 11.3% of the samples, which indicates significant contamination of the environment with tapeworm eggs [12].
Larval forms from the gastrointestinal tract enter the liver with the portal blood. Then, cysts are formed, and they may be spread to the lungs and brain. Lesions forming in the internal organs occur in the form of indurated infiltrates and are associated with connective tissue proliferation [4-6].
The disease is slowly progressive, which is associated with specific and long development of larval forms located in the liver. Depending on the species, the incubation period ranges from several months to several years (E. granulosus) and 5-10 years (E. multilocularis) [1, 4-6].
Due to the long latency period, it is impossible to determine how the infection occurred on the basis of the medical history. Risky behaviors may only be roughly specified. According to surveys conducted among patients in Germany and Austria, the greatest risk is associated with: having a dog that may come into contact with wild animals, the profession of a farmer or forester, chewing blades of grass, growing vegetables and eating strawberries. No correlation was found with the consumption of forest fruits growing close to the ground (bilberries, blueberries) or mushrooms [13].
Therefore, observing basic hygiene rules seems to be the best way to prevent infection. Heat treatment is also effective because tapeworm eggs die at temperatures above 60 degrees [13].
In humans, the transmission of infection by dogs (herding, stray and domestic) may be a serious problem. Humans become infected with the parasite via the fecal-oral route, usually through contact with infected animals or their feces containing eggs. The eggs may also be found on the fur of animals, especially around the anus, on the muzzle and paws [6].
Infection rates in the dog population are up to 31.86%, and differences in prevalence rates reflect a distribution pattern partially similar to that for the incidence in people.
Owning a dog was considered one of the highest risk factors for both E. multilocularis and E. granulosus. An increased risk was also associated with having more dogs in the family and the ease of dogs’ access to raw entrails [8].
Infected dogs were described for several decades on the island of Hokkaido in Japan, and the first case of latent invasion in a cat was also recorded there [13]. In August 2022, a case of alveolar echinococcosis in a dog was described in the Polish rural areas. Another case was described in the Pomeranian province in a 6-year-old male German shepherd in June 2023 [14].
Cases of infection in cats have been reported in Switzerland, France and the Czech Republic. However, experimental studies showed that they were not appropriate definitive hosts as parasite development was difficult and low numbers of new individuals developed, most often not reaching maturity. Therefore, cats most likely play a marginal role in tapeworm transmission [12]. Regular deworming of pets with effective and recommended preparations is necessary [12, 13].
The occurrence of the parasite in cattle and sheep also coincides rather closely with the incidence in people.
The reduction in livestock infection rates was found to correspond to a similar decrease in people. However, a more similar pattern was observed for cattle compared to sheep, suggesting its potential role as an indicator species for estimating the risk of infection in humans in areas where livestock breeding is practiced [3, 8].
Clinical picture
The embedding of larvae is asymptomatic and the parasite may reproduce for decades. Therefore, it may take many years for lesions to become apparent on imaging [3-5, 9]. In the liver, the growth rate is variable and ranges from 1 mm to 5 mm in diameter annually [9]. As mentioned above, pediatric cases are very rare. However, in the brain or in the visual organ, echinococcosis may cause symptoms at an early stage, which is why these locations are the most common in the youngest group of patients [4, 15]. With the exception of immunocompromised patients, it is estimated that the incubation period lasts 5-15 years in the majority of cases [6, 9].
If any symptoms occur, they are nonspecific. Patients most often report pain and a feeling of distension in the epigastric region, bloating, sometimes nausea.
Therefore, a significant proportion of hepatic cysts are detected incidentally on imaging, and mild manifestations often lead to an incorrect initial diagnosis and delayed implementation of suitable treatment [4]. Clinical manifestations may vary depending on the location of the cysts, which are most commonly found in the liver (over 65% are usually located in the right lobe) and lungs (25%). They may also be located in other organs: the bones, spleen, peritoneal cavity, ovaries, heart and central nervous system (CNS; in the brain and spinal cord) [4, 5, 11, 15]. Although bone involvement is very rare (< 1% of cases), it causes a severe pathology with mortality of over 50% [6]. Renal cysts (accounting for 1-4% of cases) cause a nonspecific pathology associated with lumbar pain [6].
Most primary infections are confined to a single cyst, but 20% to 40% of patients experience the involvement of more than one organ [4, 15]. The lesions may also grow large, compressing the surrounding organs and their structures. It may lead to cholestasis (manifested as symptomatic jaundice) and portal vein obstruction (which may contribute to segmental or lobar hepatic atrophy) [4, 9]. Lesions located near the hepatic veins or the inferior vena cava may cause symptoms resembling Budd-Chiari syndrome [4, 6]. Cysts situated on the periphery of the liver are the least serious and remain asymptomatic for longer periods [4, 6].
Typical symptoms of a pulmonary focus include chronic cough, chest pain, and dyspnea [15]. Other complications include ascites, splenomegaly, abscess formation, and fistula development (13-37%) [6], and the spontaneous or traumatic rupture of the cyst may lead to anaphylactic shock [4, 5, 15].
Rupture of a hydatid cyst is a rare but serious complication. According to the classification published by Lewall and McCorkell, hydatid cyst rupture may be divided into three types: closed, communicating and direct. Closed rupture refers to a situation where only the endocyst of the parasite is ruptured and the contents of the cyst remain in the host-derived pericyst. A rupture is described as communicating if the contents of the cyst escape through the bile ducts or bronchioles, which are adjacent to the pericyst. Direct rupture occurs when both the endocyst and pericyst rupture, releasing the cyst contents directly into the peritoneal or pleural cavity.
Rupture of the cyst into the peritoneal cavity is usually symptomatic and characterized by peritoneal symptoms and an acute abdomen. Fluid escaping from the echinococcal cyst or bile has the potential to cause peritonitis. The cyst content may also induce allergic reactions varying from maculopapular rash on the skin to life-threatening anaphylactic shock [16].
The symptomatic form of E. multilocularis infection is a distinct clinical issue. It is characterized by a course similar to metastatic cancer, associated with body wasting and a high mortality rate of up to 90% [1, 11]. The pathology is likely to spread if the lesion is located in the vicinity of blood vessels [6]. The spread of the disease to other organs was observed in 24.8% of patients [11].
Diagnosis
Diagnosis may pose some difficulties, especially in areas where the disease is not endemic. The diagnostic process should begin with a thorough history, including a history of exposure, contact with dogs and livestock, or immigration. Imaging and serology should be performed as the next step. Only the combination of clinical examination, imaging techniques and laboratory diagnostics may allow for a highly probable diagnosis [4, 6, 9].
Abdominal ultrasound is considered the gold standard [9] and remains the method of choice for screening due to its availability. It is also helpful in monitoring the disease after treatment. Moreover, it facilitates the imaging of peripherally located lesions in the lungs. However, it has numerous limitations. In the case of liver echinococcosis, the WHO-IWGE (Informal Working Groups on Echinococcosis) developed an ultrasound classification to determine the stage of the disease (the classification refers to E. granulosus) [4, 5, 17, 18]. Based on the classification, CE1 and CE2 stages indicate an active form, CE3 a transitional one, in which the cyst has been damaged, while CE4 and CE5 indicate an inactive disease [4, 15]. The developed WHO-IWGE PNM classification system is similar to the TNM classification of tumors. “P” refers to the extent of the hepatic location of the parasite, “N” determines the involvement of adjacent organs, and “M” refers to the absence (M0) or presence (M1) of distant metastases [9] (Tables 1 and 2).
Table 1
Classification system for human alveolar echinococcosis
| P – localization of the primary lesion | |
| PX | Primary lesion cannot be assessed |
| P0 | No detectable liver lesion |
| P1 | Peripheral lesions without proximal vascular and/or biliary involvement |
| P2 | Central lesions with proximal vascular and/or biliary involvement of one lobe |
| P3 | Central lesions with hilar vascular and biliary involvement of both lobes and/or with involvement of two hepatic veins |
| P4 | Any lesion with extension along the portal vein, inferior vena cava, or hepatic arteries and the biliary tree |
| N – extrahepatic involvement of neighboring organs or tissues1 | |
| NX | Cannot be evaluated |
| N1 | No regional involvement |
| N2 | Regional involvement of contiguous organs or tissues |
| M – absence or presence of distant metastases2 | |
| MX | Not completely evaluated |
| M0 | No metastasis |
| M1 | Metastasis present |
Table 2
Staging of alveolar echinococcosis based on PNM classification
| Stage of AE | P | N | M |
|---|---|---|---|
| Stage I | P1 | N0 | M0 |
| Stage II | P2 | N0 | M0 |
| Stage IIIa | P3 | N0 | M0 |
| Stage IIIb | P1-3 P4 | N1 N0 | M0 M0 |
| Stage IV | P4 Any P | N1 Any N | M0 M1 |
The Baltimore Centre believes that ultrasonography has a sensitivity of 85% and allows detection of cysts, as well as cyst rupture. Nevertheless, computed tomography (CT), in their experience, has a sensitivity of 100% in demonstrating cyst rupture and is mostly preferred [19].
It is often necessary, especially before planned surgery, to supplement the diagnosis with the use of more accurate imaging techniques for better evaluation, e.g., CT and magnetic resonance imaging (with the advantage of MRI due to better visualization of fluid areas) [4, 5, 9].
Serological testing for the presence of antibodies to Echinococcus spp. is a common diagnostic method. The ELISA test is used as a basic examination, followed by confirmation with the immunoblot assay [13].
Immunodiagnostics is seen as a diagnostic aid due to its limited sensitivity and specificity. A significant number of infected patients do not produce an immune response, which translates into a significant percentage of false negative results [4, 15].
The definitive diagnosis is based on the finding of protoscolices in biopsy or surgically removed material. Molecular PCR studies of the samples are also performed [13, 20].
The results of laboratory peripheral blood tests may sometimes include: increased alkaline phosphatase, with normal levels of aspartate transaminase (AST), alanine transaminase (ALT) and bilirubin, as well as eosinophilia [4, 15].
Treatment
The key concept of treatment is to adopt a multidisciplinary approach to the disease.
Three therapeutic modalities are available for the treatment of liver echinococcosis: pharmacotherapy, surgery (open or laparoscopic approach) and percutaneous surgery [4, 5]. Until the 1980s, surgery was the only therapeutic option for patients. It still constitutes the first line of treatment and is characterized by the greatest effectiveness, especially for large cysts (CE2-CE3) or for single superficial cysts, taking account of the likelihood of their spontaneous or traumatic rupture. The presence of complicated cysts (infected, communicating with the bile ducts and with the mass effect) is also an indication for surgery involving drainage rather than resection [4]. Only 20-50% of patients are eligible for surgery [4, 6]. Surgical intervention is contraindicated in patients with a contributory history, with inactive, asymptomatic cysts or multiple lesions which are difficult to access safely [4].
Surgical options may be divided into radical and conservative methods (e.g., unroofing or capitonnage). Radical procedures are associated with a lower risk of recurrence, but the surgical risk is higher. Despite the frequent involvement of adjacent lymph nodes by the parasite, studies have shown that relapse did not depend on removal, but rather on the complete resection of the hepatic cyst, further emphasizing the importance of a safe tissue margin [4, 6]. Postoperative complications include bleeding, abscess, inflammation and fistula of the bile ducts, while non-resectional procedures are associated with echinococcal recurrence [4]. Perioperative mortality ranges from 0.5% to 4% [9].
Surgical treatment should be combined with pharmacotherapy in order to reduce the risk of recurrence. However, no clear guidelines have specified the time of administration of albendazole before and after the planned surgery [4, 5, 9].
In the case of alveococcosis, pharmacotherapy is continued for at least 2 years after the surgery (in less advanced cases) or even for the rest of the patient’s life (in advanced cases) [13].
The PAIR procedure is a minimally invasive ultrasound-guided procedure implemented in the case of liver cysts that are not eligible for resection because of their size or location. It consists of four stages: percutaneous puncture of the cyst, aspiration of fluid from the cyst, injection of an agent (e.g., 95% ethanol or 20% NaCl) for at least 15 minutes, and reaspiration of the fluid [17]. It is used in combination with pharmacotherapy. The procedure carries a potential risk of anaphylactic shock [4, 5, 9, 15].
Liver transplant (LT) is used in incurable cases limited only to the liver (E. multilocularis infection). The presence of extrahepatic foci disqualifies the patient from surgery [9]. Currently, there is a shift from liver transplant to the advantage of large resections and palliative, often multi-stage, surgery. Aggressive surgical management to remove as much of the parasite as possible provides favorable conditions for adjuvant pharmacotherapy and prolongs survival in advanced cases of alveococcosis [11].
The “watch and wait” approach may be a solution for uncomplicated cysts (CE4 and CE5 stages) if the patient is provided with long-term ultrasound follow-up [4, 5, 15].
Pharmacotherapy is recommended for cysts smaller than 5 cm in the liver and lungs, in patients with lesions in two or more organs, and in inoperable patients in whom there are indications for continuous medication use [4, 15]. Albendazole is a medication of choice for continuous administration at a dose of 2 × 400 mg [4, 5]. Intermittent therapy is not recommended [4]. Albendazole treatment was found to prolong the 10-year survival of patients by approximately 80%. However, complete recovery occurred in only 1/3 of patients [9].
In Poland, conservative therapy is difficult for patients due to the high cost of recommended drugs (over PLN 1,500/month) and the lack of reimbursement [5, 11].
In untreated patients, the prognosis is poor. According to data from 1990-2011, cases of diagnosed alveolar echinococcosis in Poland resulted in the death of 19% of the patients, and their average age at death was 54.1 years. The average survival from diagnosis was 4.54 years and ranged from 2 months to 15 years [11].
Treatment radically changed life expectancy since diagnosis from 3 years in the 1970s to 20 years in 2005 [6].
The present authors’ own material
Since 2019, a total of 73 patients with liver echinococcosis have been treated in the Department of General and Transplant Surgery, including 52 women and 21 men aged 22 to 84 years. The majority of them, i.e., 27 patients, were in the 50-65 age group and 61% of the patients lived in urban areas.
On average, several new patients presented each successive year. However, a significant increase in enrolling patients (53 people) has been observed over the past 2 years. Patients referred to the department had undergone diagnostics for several months. The definitive diagnosis was made on the basis of four-phase computed tomography or contrast-enhanced electromagnetic resonance imaging, assessed in the 1st Department of Clinical Radiology of the Medical University of Warsaw. Serological tests were also performed. Five results of outpatient serological tests turned out to be false negative, so the radiological outcomes were decisive. In one case, the positive serological ELISA result and the confirmatory Western Blot test turned out to be false positive.
Eligibility for surgical treatment was based on the size and location of cystic lesions (Figs. 1 and 2) and on the clinical condition of the patients. Out of 73 patients, 30 were deemed eligible for surgery. We performed 25 liver and cyst resections (Figs. 3-5), 2 splenectomies and 3 drainage procedures (unroofing, PAIR-as can be seen in Fig. 6, with the right location of the cyst to perform the procedure). All patients scheduled for surgical treatment received albendazole from 2 to 5 months before surgery. Following cyst resections, pharmacotherapy lasted from 2 to 4 weeks, and after drainage procedures, it was definitely longer, i.e., from several months up to a year (Table 3).
Table 3
Summary of the clinic’s experience presented in the article
| Total number of patients1 – 73 (age 21-84) | ||
|---|---|---|
| Sex | Male – 21 (29%) | Female – 52 (71%) |
| Domicile | Urban areas – 45 (61%) | Rural areas – 28 (39%) |
| Treatment option | Surgical – 30 (41%)2 liver and cyst resections – 25 splenectomies – 2 drainage procedures (unroofing, PAIR) – 3 | Non-surgical – 43 (59%)3 |
Fig. 1
An operable liver echinococcal cyst (E. granulosus), magnetic resonance imaging. Echinococcal cyst in the right lobe of the liver. Multiple septa visible. Resection of the cyst possible
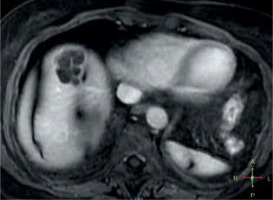
Fig. 2
An inoperable lesion (E. multilocularis), computed tomography imaging. Location in the central part of the liver. Proximity to the hepatic hilum and significant vessels
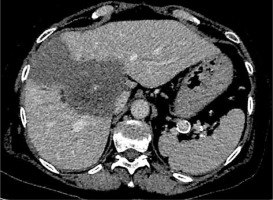
Fig. 3
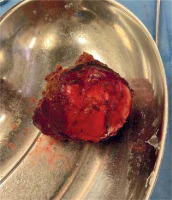
Liver cyst resection (E. granulosus), intra-operative material. Lesion of segment 7/8 of the liver. Diameter of 8 cm. Patient not receiving pharmacotherapy (albendazole) due to an attack of acute porphyria after taking the drug
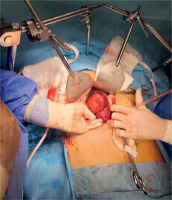
Fig. 5
A resected cyst, visible above after transection, intra-operative material. Yellow-white, gelatinous masses in the lumen of the cyst. According to the histopathological result, necrotic echinococcal elements (scolices)
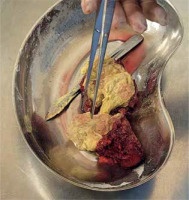
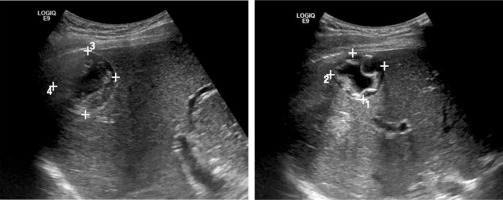
Fig. 6
Echinococcal cyst (E. granulosus) with septal elements, located subhepatically, in segment VI of the liver, ultrasound image. Diameter of 3.5 cm. Percutaneous access available
Echinococcosis was confirmed in all cases of the pathomorphological examination. E. multilocularis was found in one case. We performed 9 molecular tests, confirming the pig strain in eight cases and the sheep strain in one case.
The remaining patients were treated with albendazole. The reasons for ineligibility for surgical treatment were: the extent of the lesions, their unfavorable location, involvement of other organs or poor prognosis due to concomitant diseases.
All surgically treated patients are still alive. Complications including wound infection and biliary fistula were associated with drainage procedures. One case of extensive liver resection with Roux-en-Y hepaticojejunostomy and resection of the small intestine in a patient with alveococcosis was complicated by a biliary fistula of hepatointestinal anastomosis.
No complications developed following procedures performed in patients under 60 years of age, with a non-contributory past medical history, eligible for the resection of individual liver lesions.
In addition, we also noted one case of a unilocular echinococcal cyst located in the liver in a potential deceased organ donor.
Conclusions
According to the authors, the number of cases of liver echinococcosis is underestimated in Poland and it is frequently overlooked in the course of diagnosis.
Ultrasound diagnostics combined with serology in our opinion is insufficient. Due to the fact that ultrasonography is a subjective test, some cases may be missed, especially in the presence of a false-negative serological test. Although ultrasound is the gold standard in diagnostic imaging, contrary to recommendations, we believe that it should only be performed in a specialist center with experience in the evaluation of liver lesions.
In the vast majority of cases, multiphase contrast-enhanced computed tomography or magnetic resonance imaging at a reference center is required, mainly due to the unreliable quality of ultrasound examinations, especially those performed outside our center. In addition, it is useful for the planning of a surgical procedure.
Cyst resection with pre- and postoperative albendazole therapy is the only effective treatment option.
A large percentage of patients were found ineligible due to the inoperability of the tumor, most likely resulting from delayed diagnosis (excessive size, inappropriate location, age and poor general condition of the patients). They were found eligible for pharmacological treatment.
In patients who are ineligible for resection therapy, pharmacotherapy and/or drainage procedures are considered, but they carry a risk of numerous complications.
Mostly, a correct diagnosis is made too late.