Introduction
Successful embryo implantation depends on synchronization of a viable embryo, receptive endometrium, and maternal immunological acceptance, with a major regulatory role played by ovarian steroids [1].
In clinical practice, endometrial thickness (EMT) is a marker of endometrial receptivity and a prognostic factor in implantation. The optimal EMT for embryo transfer (ET) is assumed to be at least 7 mm or more at the end of the follicular phase [2], while the pregnancy rate in women with ≤ 6 mm of EMT was only 29.43% [3]. Another challenge is the reliability of EMT measurement as a predictor of the occurrence of pregnancies in IVF cycles because it seems to be a good factor only for the assessment of conception probability, which significantly drops below an EMT of 7 mm [4]. Other key factors such as endometrial pattern also play a vital role in successful implantation and may be as important as EMT [5]. Newly emerging diagnostic tools such as endometrial receptivity array seem to be very accurate in determining the window of implantation; pregnancies have been described even in thin endometria < 5 mm, as well as in hyperechogenic endometria [6], so endometrial assessment has become part of standard monitoring during IVF and intracytoplasmic sperm injection (ICSI) with ET treatment, and endometrial pattern, endometrial blood flow, and EMT have been regarded as prognostic factors of IVF-ICSI treatment [7].
Recently, significant work has focused on the treatment of refractory thin endometrium in ART in an effort to increase implantation and live birth rates. Regenerative medicines with stem cells, such as endometrial mesenchymal stem cells and N-cadherin+ endometrial epithelial progenitor cells within the endometrium and multipotent cells from bone marrow [8], or intrauterine infusion of platelet rich plasma (PRP), are other new modalities [9].
Platelet-rich plasma is an autologous concentration of blood plasma more than the circulating blood, which contains several growth factors and more than 800 proteins (cytokines, hormones and chemo-attractants of stem cells, macrophages, and neutrophils) [10], which have pro-regenerative, proliferative, mitogenic, angiogenic, chemotactic, proinflammatory, and antiapoptotic properties [11], thus improving the endometrial quality and implantation rate [12], and activating peripheral blood mononuclear cells, which releases IL-10, an anti-inflammatory cytokine involved in tissue regeneration. Upregulation of its expression in endometrial stromal cells improves the endometrial receptivity, making it a novel and probably successful treatment in women with thin endometrium [13].
The aim of the current study is to evaluate the effect of intrauterine PRP infusion on the day of human chorionic gonadotropin (hCG) injection on the ongoing pregnancy rate in patients with refractory thin endometrium undergoing an IVF cycle.
Material and methods
After an explanation of the nature of the study, Ethics Committee approval and written consent were obtained from the patients. This prospective, single-arm clinical trial was performed on a total of 85 infertile women; 6 cases were excluded (2 cases declined to participate, and 4 cases withdrew from the study) and 13 cases were cancelled (6 cases had poor ovarian response, 3 cases had poor quality embryos, one case had degenerated egg, and 3 cases were COVID-19 positive). All were candidates for an IVF cycle at the ART Unit of Ain Shams University Maternity Hospital in the period from January 2021 to August 2021.
Study population
Sixty-six infertile women with a refractory thin endometrium, characterized by atrophy with endometrial interface measurements below 7 mm by ultrasound on the day of hCG injection in a fresh ET cycle, which did not respond to standard medical therapies to increase the EMT in more than 2 previous cycles [14], and were candidates for an IVF cycle at the ART Unit of Ain Shams University Maternity Hospital.
Inclusion criteria
Age 20–35 years, with EMT < 7 mm on the day of hCG injection in fresh IVF cycles, normal hysteroscopic and hysterosalpingographic results, level of day 2 follicle-stimulating hormone (FSH) < 10 IU/l, body mass index (BMI) between 18 and 28, and primary or secondary infertility.
Exclusion criteria
Patients with any other known cause of implantation failure, patients with past history of any medical disorder (systemic, immunological, endocrine, and haematological disease) and those using gonadotropins or hormonal contraception during the latest 6 months, patients with tubal factors (hydro or pyosalpinx), patients with poor embryo quality, and patients with anatomical uterine abnormalities.
All participants were submitted to the following: Careful and detailed history, general (vital signs: pulse, BP, basal body temperature charting), BMI was calculated, head and neck examination, breast and abdominal examinations, and local examinations (P/V), baseline transvaginal ultrasound, hysteroscopic examination, hysterosalpingogram, and if indicated, laparoscopy and full required laboratory tests were requested.
Study intervention
Induction of ovulation protocol
In long luteal phase, gonadotrophins-releasing hormone agonist (GnRHa) protocol:
S.C. GnRHa injection as (Decapeptyl 0.1 mg triptorelin, Ferring, Switzerland) was started daily from day 21 of the preceding cycle until the day of hCG [15];
on day 2–3 of the cycle down-regulation was confirmed by TV U/S checking EMT < 5 mm and D2 hormonal profile (E2 < 80 pg/ml and LH < 5 IU/l). Then, ovarian stimulation was performed using gonadotrophins (Gonapure 75 I.U). The starting dose of gonadotrophins was prescribed according to age, BMI of the subjects, anti-Mullerian hormone (AMH) level, and antral follicle count [16]. The dose was adjusted according to sonographic response of the ovaries and E2 level [15];
ovarian response was assessed by TV folliculometry from D8 and then every day until the moment the leading follicle reach 15 mm. Daily TV U/S was performed until the largest follicle reached a diameter > 17 mm with good count of secondary follicles, serum oestradiol > 200 pg, and progesterone concentration < 1.5 ng/ml were documented. Intramuscular hCG was administered (Choriomon 10,000IU);
on the day of hCG injection, serum E2 level and serum progesterone (P4) were tested, and EMT and pattern (day 1) were assessed. Thirty-six hours after hCG injection, ovum pick up was performed;
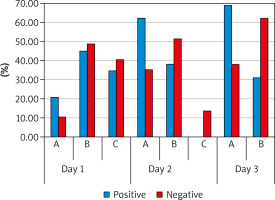
endometrial pattern was classified according to the morphology (Zhao’s grading) as follows: pattern A – a triple-line pattern consisting of a central hyperechoic line surrounded by 2 hypoechoic layers; pattern B – an intermediate isoechogenic pattern with the same reflectivity as the surrounding myometrium and a poorly defined central echogenic line; or pattern C – homogenous hyperechogenic endometrium [17].
Preparation and infusion
Intrauterine infusion of preparation and infusion (PRP) was carried out at the day of hCG injection under ultrasound guidance. Platelet-rich plasma was prepared from autologous blood, following all antiseptic precautions at room temperature:
approximately 8.5 ml of peripheral venous blood was drawn from the patients using 10 ml syringes;
samples were anticoagulated immediately in a sterile tube containing citrate dextrose adenine then mixed and agitated gently (inversion/resuspension);
this was followed by immediate centrifugation and fractionation of each blood sample into PRP at the top and red blood cells at the bottom. The platelet concentration was determined using an automatic cell-counting system; this process yielded platelets in concentration higher than the concentration in circulating blood;
0.5–1 ml of PRP layer at the top was aspirated carefully to be infused immediately into the uterine cavity with a Labotec catheter (Classic Embryo Replacement Catheter, Gottingen, Germany) under ultrasound guidance.
Oocyte retrievals
Transvaginal ultrasound-guided ovum pick-up was performed 34–36 h after hCG injection:
after administration of the chosen anaesthetic agent, patients were placed in the dorsal lithotomy position and a sterile speculum was inserted into the vagina and rinsed with saline and betadine. The vagina was rinsed with saline, the speculum was removed, and a vaginal ultrasound probe with needle guide attached (17-gauge) was inserted into the vagina passing through its wall and into the follicles;
a vacuum pump pressure was activated at ±180 mm Hg according to the manufacturer’s instructions, and then follicular fluid and oocytes were aspirated into warmed tubes;
the tubes were then placed in a tube warming rack in the embryology laboratory;
the content of the tubes was poured into culture, plates and the oocytes were identified, on oocyte retrieval day EMT and pattern were assessed and recorded (day 2). During this cycle, luteal phase support was started by daily intramuscular injection of 100 mg progesterone, combined with 200 mg vaginal progesterone soft capsules twice daily.
Embryo transfer
Good-quality blastocysts were transferred on day 5 after oocyte retrieval. Blastocysts scored as grade 5AA or 6AA were considered as good quality (according to the Gardner and Schoolcraft scoring system) [18]:
all transfers were performed under ultrasound guidance by one expert infertility subspecialist using a soft transfer catheter to the mid portion of the uterine body for subsequent implantation. Embryo transfer was performed according to the 2013 American Society for Reproductive Medicine (ASRM) guidelines (Practice Committee of the ASRM and the Practice Committee of the Society for Assisted Reproductive Technology, 2013). Also, endometrial pattern and thickness were reassessed before ET (day 3);
patients were placed in the dorsal lithotomy position, and a speculum was placed into the vagina until the cervix was in view, which was prepared with saline solution, and excess cervical mucous was aspirated with a syringe or wiped with sterile cotton swabs;
the serum hCG level was tested 14 days after ET, and if it was positive, hormone supplements were continued until 12 weeks of gestation;
vaginal ultrasonography was performed 35 days after transfer in cases of a biochemical pregnancy, and clinical pregnancy was confirmed by the presence of an intrauterine foetal heart beat or gestational sac (GS). That was followed up until 20 weeks of gestation to observe the pregnancy outcomes.
All procedures were done by supervisors and experts.
Assessment of the patients
On the 13th to 14th day after the ET, chemical pregnancy was confirmed by measuring the level of b-hCG (β-hCG titre more than 50 mIU/ml). Also, the implantations were approved by dividing the number of observed embryonic sacs in the 6-week-old sonogram by the number of the transferred embryos. The rate of clinical pregnancy was recorded by dividing the number of foetal poles with an observed heartbeat in the 6-week-old sonogram or GS by the number of the transferred embryos. The miscarriage rate was expressed per clinical pregnancy cycle as spontaneous pregnancy loss after sonographic visualization of an intrauterine gestational sac. Twenty weeks of gestation was considered as ongoing pregnancy.
Outcome measures
Primary outcome: ongoing pregnancy rate in patients with refractory thin endometrium undergoing an IVF cycle.
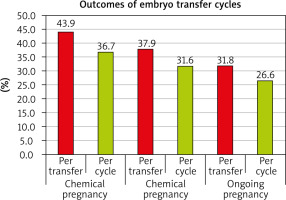
Secondary outcomes: chemical and clinical pregnancy rates, in addition to the EMT and pattern at the time of oocyte retrieval and ET.
Statistical analysis
Data were analysed using IBM© SPSS© Statistics version 23 (IBM© Corp., Armonk, NY). Normally distributed numerical data were presented as mean and standard deviation, and skewed data were shown as median and interquartile range. Qualitative data were presented as number and percentage. Comparison of normally distributed numerical data was done using the unpaired t-test. Skewed data were compared using the Mann-Whitney U test. Categorical data were compared using the χ2 test or Fisher’s exact test, if appropriate. P < 0.05 was considered statistically significant, and OR and RR were calculated.
Results
A total number of 85 infertile women with a refractory thin endometrium were recruited. Six cases were excluded (2 cases declined to participate and 4 cases withdrew from the study), and 13 cases were cancelled (6 cases had poor ovarian response, 3 cases had poor quality embryos, one case had degenerated egg, and 3 cases were COVID 19 positive on the day of OR). Hence, our study was carried out on 66 patients with the same inclusion and exclusion criteria.
Twenty-nine out of 66 cases had positive chemical pregnancy.
The following tests were done:
independent-samples t-test of significance was used when comparing 2 means;
Mann-Whitney U test: for 2-group comparisons in non-parametric data;
paired samples t-test of significance was used when comparing between related samples;
χ2 test of significance was used to compare proportions between qualitative parameters;
the confidence interval was set to 95%, and the margin of accepted error was set to 5%. So, the p-value was considered significant as the following:
– p < 0.05 was considered significant,
– p < 0.001 was considered as highly significant,
– p > 0.05 was considered insignificant.
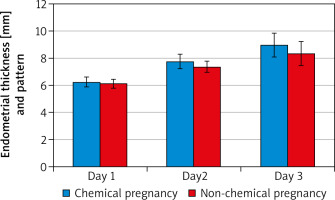
There was a statistically significant increase in EMT “mm” after intrauterine PRP infusion. On D2, mean EMT (7.75 ±0.48) ranged 5.5–9 and D3 mean EMT (8.97 ±0.65) ranged 5.5–11 vs. D1 mean EMT (6.19 ±0.34) ranged 4–6.8. It seemed that PRP could expand EMT with its high content of growth factor. Also, there was a highly statistically significant difference between D2, D3 patterns compared to D1 pattern with p-value (p < 0.001). on D1 15.2%, 47%, and 37.9% of patients had patterns A, B, and C, respectively, vs. D2 – 47%, 45.5%, and 7.6%, respectively, and D3 – 51.5% and 48.5%, respectively.
Multivariate analysis revealed that significant predictors of chemical pregnancy were EMT and endometrial pattern, which were the best independent predictors of chemical pregnancy with OR 2.452 (95% CI: 0.674–8.924); p-0.037 and OR 2.869 (95% CI: 0.789–10.441); p-value 0.043, respectively.
Discussion
The current study showed that intrauterine PRP infusion in women with refractory thin endometrium undergoing IVF resulted in an increase in ongoing pregnancy, chemical pregnancy, clinical pregnancy, and implantation rates. Endometrial thickness and pattern showed significant improvement in days 2 and 3 in cases who had chemical pregnancy as opposed to those who did not. There were no significant differences in age, BMI, type or duration of infertility, number of previous ART cycles, or hormonal profile between those who had positive chemical pregnancy and those with negative pregnancy.
The current study results agreed with the results of the studies done by Nazari et al. [19], Kusumi et al. [20], and Eftekhar et al. [21]. The first study was a double-blinded randomized controlled study including 60 women with a history of a cancelled frozen ET cycle due to thin endometrium, who were randomized to either PRP or sham catheter and showed a significant difference in favour of PRP (after 2 infusions – the first on day 11 or 12 and the second 2 days later) in terms of endometrial thickness, and chemical and clinical pregnancy rates. The second study was a single-arm self-controlled trial involving 36 patients with thin endometrium and repeated implantation failure undergoing frozen ET in a second hormone replacement therapy (HRT) cycle, who received PRP intrauterine infusion on days 10 and 12 of the cycle and found a significant increase in EMT at day 14 of the cycle, concluding that this significant increase in the EMT could possibly increase the pregnancy rate. The third was a randomized controlled trial involving 83 women with poor endometrial response to standard HRT, who were randomized into 2 groups on day 13 of a frozen/thawed ET cycle to receive either PRP and HRT or HRT only. If EMT did not increase after 2 days, another PRP infusion was done, and when the EMT reached 7 mm or more ET was done, a significant increase in endometrial thickness, chemical pregnancy, clinical pregnancy, ongoing pregnancy, and implantation rates was found in the PRP group as compared to the control group, concluding that PRP may be effective in improving the growth of the endometrium and possibly improving the pregnancy outcomes. However, Tehraninejad et al. [22] in a non-randomized controlled trial including 85 patients with recurrent implantation failure and normal endometrium found no significant difference between 2 rounds of PRP infusion 2 days apart and controls in terms of endometrial thickness, and chemical, clinical, and ongoing pregnancy rates; however, this may be due to choosing cases with normal endometrium in this study.
Tandulwadkar et al. [23] revealed the role of PRP and its biostimulation effects on the endometrium microvascular endothelium in improving the endometrial thickness, vascularity, and receptivity in women with suboptimal EMT, enhancing the implantation rate and reducing the incidence of ET cycle cancellation. Being an autologous resource, and therefore harmless to the patient, easy to obtain, and of very low cost, it is recommended for inclusion in the different protocols for endometrial preparation in ART, even in those with refractory endometrium [12].
The advantages of the current study include its prospective single-arm clinical nature with minimal bias as every effort was made to ascertain that all follow-up data were correct, and only complete information was included in the data analysis, and all clinical assessment ovulation induction and assessment of study outcomes were done by the same team. Being an autologous resource, therefore harmless to the patient, easy to obtain, of very low cost and contains a high concentration of growth factors (growth factors were obtained by genetic and recombi-nant engineering procedures; being expensive and repeated doses were required to achieve an optimal therapeutic effect) and the type of used platelets concentration in PRP was defined. The limitations of the current study included the COVID-19 pandemic, the relatively small sample size, exclusion and cancellation of many cases, and the absence of a control group receiving a placebo. Thus, the changes of EMT were observed only before and after infusion and between subgroups of women who conceived or did not, exclusion and cancellations of many cases, it was done on fresh cycle only, using luteal phase GnRHa protocol and the unknown genetic composition of embryos that were used in the study (as it was unusual to do pre-implantation genetic testing as a routine), despite using morphologically good-quality blastocysts. Embryo morphology might not represent the real quality of embryos.