Introduction
Chrysanthemum (Chrysanthemum morifolium ) is known as an ornamental plant that has a variety of flower shapes, sizes, and colors (Wang et al., 2015; Won et al., 2018). The global count of chrysanthemum cultivars has been estimated to exceed 20000 (Wang et al., 2014). According to traditional Chinese medicine, chrysanthemum plants have properties that enhance vision and liver function, reduce inflammation, prevent fatigue, and address conditions such as fever, headache, eye redness, and swelling caused by toxins (Liu et al., 2015). Chrysanthemum plants contain various organic compounds such as alkanes, flavonoids, terpenoids, unsaturated fatty acids, and polysaccharides (Chae, 2016). Flavonoids, a class of polyphenolic compounds, are synthesized in various plant parts, such as fruits, grains, barks, roots, stems, leaves, and flowers (Panche et al., 2016). Extensive pharmacological studies and clinical practice have demonstrated the robust biological activity of flavonoids, including antibacterial, antiviral, anticancer, hepatoprotective, anti-inflammatory, and antioxidant properties (Kumar et al., 2013; Geçibesler, 2017; Wang et al., 2018). Flavonoids have been linked to a wide range of health-promoting effects and are an essential component in a wide range of nutraceutical, pharmaceutical, medicinal, and cosmetic applications. This is due to their ability to modulate key cellular enzyme functions, as well as their antioxidative, anti-inflammatory, antimutagenic, and anticarcinogenic properties (Panche et al., 2016). Flavonoids have been widely investigated for their free radical scavenging properties, which contribute to the prevention of cancer (Zhao et al., 2019; Berk et al., 2022) and cardiovascular diseases (Rees et al., 2018; Russo et al., 2019). Polyphenolic compounds, including flavonoids, can serve as supplements in the prevention and treatment of cardiovascular and inflammatory diseases (Subin et al., 2020; Chen and Zhang, 2021). Additionally, flavonoids exhibit immunostimulatory effects and can mitigate allergic responses (Hayat et al., 2017).
C. morifolium flowers are rich in several types of flavonoids, especially luteolin-7-glucoside and quercitrin (Yang et al., 2018; Han et al., 2019). Quercitrin, also known as quercetin-3-O-rhamnoside, is a glycoside derived from quercetin and deoxyrhamnose sugar (Boots et al., 2008; Li et al., 2016). This compound has been associated with antioxidant properties and potential health benefits, such as anticancer effect (Khan et al., 2016; Fernández-Palanca et al., 2019), anti-inflammatory (Ferraz et al., 2021; Neamtu et al., 2022), neuroprotective activities (Khan et al., 2019), and reduce the risk of developing cardiovascular disease (Cao et al., 2019; Jia et al., 2019). Quercetin has been found to alleviate asthma symptoms by reducing the number and activation of inflammatory cells. Additionally, it exhibits anti-viral activity against various viruses such as herpes simplex type 1, parainfluenza type 3, respiratory syncytial virus, pseudorabies virus, and Sindbis virus (Kumar et al., 2017). Moreover, quercetin demonstrates antibacterial effects against several bacterial strains, particularly those affecting the gastrointestinal, respiratory, urinary, and dermal (skin) systems (Kumar et al., 2017). This compound also exhibits preventive or risk-reducing properties for various diseases, including osteoporosis, lung cancer, and cardiovascular disease (David et al., 2016). Furthermore, it has shown beneficial effects in neurodegenerative conditions such as Alzheimer’s disease (Joseph and Muralidhara, 2013).
Conventionally, bioactive compounds are obtained by directly extracting them from plant organs. However, this approach has drawbacks as it continuously affects the availability of plant species. In addition, large-scale cultivation of plants is required, and the extraction, isolation, and purification processes can incur significant costs (Chattopadhyay et al., 2002; Pollastro and Minassi, 2021). Tissue culture offers a controlled environment that can be optimized for the production of specific secondary metabolites. Therefore, plant tissue culture presents a promising and efficient alternative method to enhance the production of secondary metabolites in larger quantities than those found in the parent plant (Madanakumar et al., 2017). One commonly employed type of culture for in vitro production of secondary metabolites is callus culture. Callus culture of Bellis perennis has demonstrated effectiveness in increasing the production of various secondary metabolites such as rutin, hesperidin, chlorogenic acid, caffeic acid, ferulic acid, rosmarinic acid, p-coumaric acid, and luteolin (Biol et al., 2016). Similarly, the callus culture of Duroy macrophylla has been shown to increase the production of terpenoid compounds (Zanca et al., 2016). In the case of Ocimum sp., callus proliferation for just 1 week resulted in higher levels of antioxidant compounds such as ascorbic acid, botulinic acid, gallic acid, carotene, flavonoids, and phenolics (Kasem, 2017).
The production of secondary metabolites through in vitro culture technology still faces various biological limitations, primarily the relatively low levels of secondary metabolites produced in plant cell cultures (Biol et al., 2016). The biosynthesis of plant secondary metabolites is influenced by environmental stress, but the accumulation of desired metabolites can be stimulated by the application of specific precursors and elicitors (Wang et al., 2015). The synthesis and accumulation of secondary metabolites in cell and organ cultures can be triggered by the application of elicitors into culture media (Naik and Al-Khayri, 2016; Rora et al., 2020).
Elicitors, which can be natural or synthetic compounds from biotic or abiotic sources, are introduced in low concentrations into living cell systems to initiate or enhance the biosynthesis of specific metabolites and play an important role in the adaptation of plants to environmental stresses. They also play a crucial role in plant defense responses against pathogen attacks (Thakur and Sohal, 2013; Naik et al., 2016; Jamiołkowska, 2020), making them one of the most effective strategies to increase secondary metabolite production (Narayani and Srivastava, 2017; Pollastro and Minassi, 2021).
Osmotic pressure, as an environmental stress factor, can alter the physiological and biochemical properties of plants and affect the concentration of secondary metabolites in plant tissues (Al-Khayri and Al-Bahrany, 2002; Naik et al., 2016). Osmotic pressure, or water stress, falls under the category of physical elicitors (Ruiz-García and Gómez-Plaza, 2013; Seraj et al., 2021). Sucrose is commonly used as the primary carbon source in in vitro culture media, but it can also act as an osmotic agent, inducing osmotic stress in plants (Reyes-Díaz et al., 2020).
The synthesis of hypericin and hyperforin compounds in Hypericum perforatum plants is greatly affected by water imbalance and osmotic pressure (David et al., 2016; Naik et al., 2016; Kladar et al., 2017). Moreover, increased levels of sucrose in growth media have been found to induce anthocyanin biosynthesis in callus cultures of Malus domestica (Liu et al., 2017) and Torenia fournieri (Naing et al., 2021). In Brassica oleracea var. italic, the addition of sucrose at specific concentrations significantly enhanced the content of sulforaphane, anthocyanin, ascorbic acid, and glucosinolates (Guo et al., 2011).
Successful elicitor application to increase the content of secondary metabolites in elicited callus or plant cell cultures depends on factors such as the concentration of the elicitor and the appropriate harvest time of the culture. Previous research has demonstrated that differences in sucrose elicitor concentration and harvest time in callus cultures of Boerhaavia diffusa L. affected callus growth and increased the production of secondary metabolites, such as β-sitosterol compounds (Patil and Bhalsing, 2016).
The purpose of this research was to investigate the impact of sucrose as an elicitor in the callus culture of C. morifolium, specifically targeting the stimulation of quercetin-3-O-rhamnoside (quercitrin) production. Additionally, two different harvest times of callus cultures, specifically 15 and 30 days, were tested to evaluate their influence on the outcomes.
Materials and methods
Materials
The materials utilized in this study included agarose, distilled water, 70% alcohol, acetic acid, acetonitrile, 1N KOH, Murashige and Skoog (MS) media (PhytoTechnology Laboratories®), cellulose filter membranes with a size of 0.22 μm, methanol p.a (Emsure), spirtus, standard quercetin-3-O-rhamnoside (quercitrin) (SIGMA®), sucrose p.a. (Merck®), and 2,4-dichlorophenoxyacetic acid (2,4-D) (SIGMA®). The plant material (C. morifolium var. Yulimar) was obtained from the Horticultural Seed and Crop Development Center in Pasir Banteng, West Java, Indonesia.
Research design, media preparation, and explants planting
This research used a completely randomized design with four different sucrose concentration treatments: 0, 30, 45, and 60 g/l. Each treatment was repeated eight times. Sucrose was added to the MS medium (Murashige and Skoog, 1962) along with 4 ppm of 2,4-D. The pH of the culture media was adjusted to 5.8 using either 0.1 N HCl or 0.1 N NaOH solution and then autoclaved at 121 °C and 1 atm pressure for 20 min.
The calli used in this study were obtained from 45-day-old chrysanthemum plantlet leaves, induced on MS medium containing 4 ppm 2,4-D. One gram of callus was subcultured onto the MS media supplemented with 2,4-D and treated with the four different sucrose concentrations. The cultures were then incubated in a culture room with a temperature range of 18–22°C and a light intensity of 2000 lux. The cultures were harvested at 15 and 30 days after planting (DAP) for parameter observation and analysis.
Observation of morphological parameters and callus growth
In this research, observations were conducted on the 15th and 30th days of the callus culture, which were considered as the harvest times or DAP. Several parameters were measured, including the morphology characteristics such as the color and texture of the callus. The growth of the callus was also assessed by measuring the wet weight, dry weight, and diameter of the callus.
The callus diameter was measured by measuring the longest side of the callus, following the method described by Ubudiyah and Nurhidayati (2013). Wet and dry weights of the callus were measured using an analytical weight. The dry weight of the callus was determined by drying it at a temperature of 50°C until a constant weight was achieved. The color and texture of the callus were determined visually, based on visual observations made by the researchers.
Extraction of callus for HPLC analysis
In the extraction process, 0.1 g of dried callus was used. It was mixed with a 4 ml solution of methanol, acetic acid, and distilled water in a ratio of 100 : 2 : 100. The mixture was then subjected to centrifugation at 100 rpm for 1 h at room temperature. After centrifugation, 2 ml of the extract was taken out and subjected to another centrifugation at 2000 rpm for 10 min. This second centrifugation step helped to further clarify the extract.
The final solution that was acquired after two centrifugations was then filtered through a 0.22 μm pore-size cellulose filter membrane. The filtrate was then used for HPLC analysis (Moghaddasian et al., 2012). All procedures were conducted at room temperature, except for the drying process of the callus.
Preparation of quercitrin standard solution
The authors prepared a standard stock solution of quercetin-3-O-rhamnoside (quercitrin) in various concentrations (50, 100, 150, and 200 ppm) using methanol as the solvent. Subsequently, the standard solution underwent filtration utilizing a 0.22 μm pore size membrane filter before being directly injected into the HPLC system (Moghaddasian et al., 2012).
HPLC analysis
The analysis of quercitrin content in the extract was performed using HPLC with a C18 column (4.6 ×250 mm) as the stationary phase. The mobile phase consisted of a mixture of methanol:acetonitrile:aquadest (10 : 10 : 75) containing 5% acetic acid as component A, and methanol as component B. A UV detector was employed, operating at a wavelength of 368 nm, with a flow rate of 1 ml/min and an injection volume of 20 μm. The chromatographic peaks were evaluated by comparing retention times and UV spectra using reference standards Moghaddasian et al. (2012).
Data analysis
The obtained data were subjected to statistical analysis using a two-way analysis of variance (Two-way ANOVA) at a 95% confidence level (α = 0.05). In the case of a significant effect (P < 0.05), further analyses were conducted using Duncan’s multiple distance test. However, descriptive analysis was employed for the data related to callus color and texture.
Results and discussion
Effect of sucrose and harvest time differences on morphology and callus growth of chrysanthemum
Callus morphology
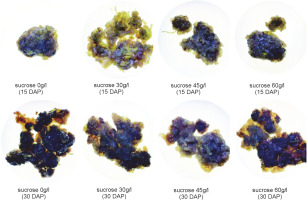
Callus morphology serves as a parameter for assessing the regeneration capacity of the callus (Sharma and Sutradhar, 2017). Visual observations of callus morphology, including color and texture, were conducted on the 15th and 30th days after planting. The color of the callus formed varied across treatments, with the majority exhibiting a brown hue (Fig. 1). Differences in callus color indicate variations in the quality, growth, and developmental stages of each callus. Callus color can range from light yellow, dark yellow, light green, dark green, yellowish green, white, greenish-white, and brownish white, to light brown, dark brown, and gray (Ożarowski, 2011; Zeng et al., 2011; Subramaniyan et al., 2014; Nurzaman et al., 2022).
Figure 1 illustrates that the majority of obtained calli exhibited various shades of brown. The browning of callus is attributed to the accumulation and oxidation of phenolic compounds in plant tissues and culture media. Phenolic compounds are produced or released in significant quantities as a defense mechanism, particularly in response to injury or exposure to stressful conditions (Jones and Saxena, 2013).
Browning reactions in plant tissue culture are closely associated with the activity of phenylalanine ammonialyase (PAL) (Cai et al., 2020), an enzyme that plays an important role in the biosynthesis of phenolic compounds (Villarreal-García et al., 2016). PAL activity can be enhanced by exogenously administered sucrose in the media (Morkunas et al., 2005). Browning can also serve as an indication of the aging process (senescence) or physiological decline in callus cells. Prolonged culture incubation (30 days after planting) resulted in the formation of darker or dark brown calli (Table 1). The dark brown color of the callus may also indicate the initiation of secondary metabolite synthesis by the cells (Souza et al., 2014).
Table 1
Two-way ANOVA table of calli fresh weight of chrysanthemum that were cultured in various concentrations of sucrose and harvested at different DAP
| Dependent variable: Calli fresh weight | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Sig. |
| Corrected model | 10.687a | 7 | 1.527 | 1996.906 | 0.000 |
| Intercept | 125.785 | 1 | 125.785 | 164517.008 | 0.000 |
| Sucrose conc. | 7.641 | 3 | 2.547 | 3331.327 | 0.000 |
| DAP | 2.298 | 1 | 2.298 | 3005.453 | 0.000 |
| Sucrose * DAP | 0.748 | 3 | 0.249 | 326.302 | 0.000 |
| Error | 0.043 | 56 | 0.001 | ||
| Total | 136.515 | 64 | |||
| Corrected total | 10.730 | 63 | |||
The presence of green color in the callus indicates the presence of chlorophyll in the tissue. Callus harvested on the 15th day exhibited a higher abundance of green color compared to those harvested on the 30th day after planting.
The presence of green color in the callus indicates the presence of chlorophyll in the tissue. Callus harvested on the 15th day exhibited a higher abundance of green color compared to those harvested on the 30th day after planting. The pigment content in callus can also vary with culture duration, as callus cells undergo changes in metabolism and growth over time (Tarrahi and Rezanejad, 2013). Previous research by Laukkanen et al. (2000) reported a decrease in the levels of green (chlorophyll) and yellow (carotenoid) pigments with the increase in culture time of Scots pine (Pinus sylves-tris L.). Generally, a decline in chlorophyll levels occurs when the culture is incubated for more than 2 weeks (Sari et al., 2018). In this study, all control calli grown on media without additional sucrose exhibited a green color, while the addition of 30 and 60 g/l sucrose to the media resulted in the degradation of chlorophyll (green pigment) in callus tissue at 30 DAP. This suggests that the administration of sucrose in the culture media can inhibit chlorophyll synthesis with varying degrees of inhibition, depending on the plant species (Sari et al., 2018).
In all treatments, the calli exhibited an intermediate texture characterized by a combination of compact and friable components. These results indicate that neither the addition of various sucrose concentrations nor the variation in harvest time had a significant impact on the formation of callus texture in chrysanthemum (C. morifolium). These findings align with a previous study that reported the lack of effect of sucrose application on the callus texture of Justicia gendarussa (Wahyuni et al., 2020).
Growth of chrysanthemum callus
The growth parameters analyzed in this research included the wet weight, dry weight, and diameter of the callus. Figure 2 illustrates the impact of sucrose application in the culture media on the wet and dry weights of the callus harvested at the 15th and 30th DAP.
Fig. 2
Fresh weight (A) and dry weight (B) of chrysanthemum calli that were cultured in different sucrose concentrations and harvested at 15 and 30 DAP
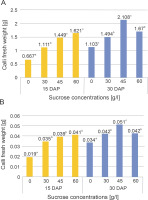
Two-way ANOVA statistical analysis (Tables 1 and 2) indicates that the application of sucrose significantly influenced the fresh and dry weights of the calli at both the 15th and 30th DAP. Duncan’s multiple distance test further revealed differences in the effects of the treatments on the fresh and dry weights of the calli, as depicted in Figure 2.
Table 2
Two-way ANOVA table of calli dry weight of chrysanthemum that were cultured in various concentrations of sucrose and harvested at different DAP
| Dependent variable: Calli fresh weight | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Sig. |
| Corrected model | 0.005a | 7 | 0.001 | 502.632 | 0.000 |
| Intercept | 0.092 | 1 | 0.092 | 67648.737 | 0.000 |
| Sucrose conc. | 0.003 | 3 | 0.001 | 761.158 | 0.000 |
| DAP | 0.001 | 1 | 0.001 | 902.632 | 0.000 |
| Sucrose * DAP | 0.000 | 3 | 0.000 | 110.772 | 0.000 |
| Error | 0.007 | 56 | 0.002 | ||
| Total | 0.097 | 64 | |||
| Corrected total | 0.005 | 63 | |||
Tables 1 and 2 demonstrate that sucrose application led to a significant increase in the fresh and dry weights of the calli at both the 15th and 30th DAP. In addition, the interaction between sucrose concentration and harvest time significantly affected the increase in callus weight. The highest average fresh and dry weights on the 15th day after planting were observed in the 60 g/l sucrose treatment (1.621 and 0.041 g, respectively), while on the 30th day, the highest average fresh and dry weights were obtained in the 45 g/l sucrose treatment (2.108 and 0.051 g various concentrations and harvesting times influenced the biomass accumulation and production of bioactive compounds in Centella asiatica (L) callus. respectively). These results indicate that the fresh and dry weights of calli grown in media supplemented with various sucrose concentrations increased with a longer harvest time. Previous research by Reddy et al. (2013) demonstrated that the supplementation of elicitors at
Biomass production in plant culture serves as an indication of optimal nutrient absorption from the culture media, which acts as a source for cellular metabolic activity (Nadirah et al., 2019). Sucrose plays a dual role as an external energy source and in maintaining osmotic pressure in the environment, thereby promoting growth in various types of plants in vitro (Cells et al., 2023). Increased osmotic pressure in the tissue facilitates water absorption, solute uptake, and the assimilation of growth regulators from the growth medium, leading to a high frequency of shoot regeneration (Yildiz et al., 2016). Therefore, osmotic pressure is crucial for cell growth and proliferation in plant tissue culture propagation. Importantly, it has been reported that high sucrose concentrations result in a significant increase in osmotic pressure (de Santana et al., 2011). Moreover, low water potential in the culture medium can reduce transpiration rates and subsequently hinder nutrient absorption. High concentrations of sucrose can induce cell plasmolysis, inhibit metabolic activity, and suppress the growth rate of the culture (Volk and Caspersen, 2017; Nurokhman et al., 2019).
The addition of various sucrose concentrations to the media had a significant impact on the callus diameter at both harvest times, as indicated by the results of the two-way ANOVA presented in Table 3. The findings from Duncan’s multiple distance test, which aimed to determine the differences in treatment effects on callus diameter, are presented in Figure 3.
Table 3
ANOVA table of calli diameter of chrysanthemum that were cultured in various concentrations of sucrose and harvested at different DAP
| Dependent variable: Calli diameter | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Sig. |
| Corrected model | 12.152a | 7 | 1.736 | 12.175 | 0.000 |
| Intercept | 396.060 | 1 | 396.060 | 2777.779 | 0.000 |
| Sucrose concentration | 7.327 | 3 | 2.442 | 17.129 | 0.000 |
| DAP | 4.197 | 1 | 4.197 | 29.438 | 0.000 |
| Sucrose concentration* DAP | 0.628 | 3 | 0.209 | 1.467 | 0.233 |
| Error | 7.985 | 56 | 0.143 | ||
| Total | 416.196 | 64 | |||
| Corrected total | 20.136 | 63 | |||
Fig. 3
Diameter of chrysanthemum calli that were cultured in various concentrations of sucrose and harvested at different DAP
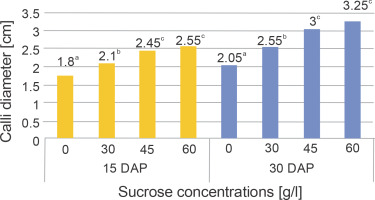
Table 3 demonstrates a significant increase in callus diameter with increasing sucrose concentration at both the 15th and 30th DAP. However, there was no interaction observed between sucrose concentration and harvest time in relation to callus diameter. The highest average callus diameter on the 15th and 30th DAP was observed in the 60 g/l sucrose treatment (2.55 and 3.25 cm, respectively). A previous study has highlighted that sucrose, being a disaccharide carbohydrate, acts as a highly soluble transporter molecule capable of efficiently crossing the plasma membrane (Peng et al., 2020). Carbohydrates play a crucial role in controlling plant morphogenesis in vitro, influencing processes such as cell enlargement, the formation of a firm cellular structure, and the composition of cell wall formation (Baskaran and Jayabalan, 2016).
Effect of sucrose on quercetin-3-o-rhamnoside (quercetrin) content of chrysanthemum callus based on harvest time differences
The results of two-way ANOVA, as presented in Table 3, indicate a significant effect of sucrose application on the quercetin content of the calli on both the 15th and 30th days after planting. Furthermore, there was a significant interaction observed between sucrose concentration and harvest time, highlighting the combined influence of these factors on callus weight.
Table 5 displays the quercetin-3-O-rhamnoside (quercitrin) content in chrysanthemum calli following sucrose treatments, at two different harvesting times (15 and 30 DAP). On the 15 DAP, the highest quercitrin content observed was 0.316 mg/g DW, representing a 27.94% increase compared to the control, which was obtained from the 60 g/l sucrose treatment. Similarly, on the 30 DAP, the highest quercitrin content recorded was 0.437 mg/g DW, demonstrating a 63.67% increase compared to the control, derived from the 45 g/l sucrose treatment.
Table 4
ANOVA table of the effect of sucrose and harvest time on quercetin content of chrysanthemum callus culture
| Dependent variable: Quercetin content | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Sig. |
| Corrected model | 0.275a | 7 | 0.039 | 6415.619 | 0.000 |
| Intercept | 7.032 | 1 | 7.032 | 1148882.776 | 0.000 |
| Sucrose concentration | 0.125 | 3 | 0.042 | 6814.222 | 0.000 |
| DAP | 0.128 | 1 | 0.128 | 20969.253 | 0.000 |
| Sucrose concentration* DAP | 0.021 | 3 | 0.007 | 1165.805 | 0.000 |
| Error | 0.000 | 56 | 6.121E−6 | ||
| Total | 7.307 | 64 | |||
| Corrected total | 0.275 | 63 | |||
Table 5
The content quercitrin of chrysanthemum callus that were cultured in several sucrose concentrations and harvested at a different time of DAP
The two-way ANOVA results reveal that the addition of sucrose significantly influenced the content of quercetin-3-O-rhamnoside in chrysanthemum callus at both 15 and 30 DAP. These findings indicate that the addition of sucrose as an elicitor in the culture media significantly increased the quercitrin content in the callus. The differences between sucrose treatments at either 15 or 30 DAP, as analyzed using Duncan’s multiple range test, are indicated by distinct letters in column 3 of Table 4.
The quercitrin content of the callus decreased when treated with 45 g/l sucrose at 30 DAP. However, in general, the quercitrin content in chrysanthemum callus increased with higher sucrose concentrations and longer harvest times (Table 5). These findings suggest that the efficacy of the elicitor is influenced by its concentration at different harvest times. Previous research has indicated that elicitor concentration is a limiting factor in secondary metabolite synthesis (Halder et al., 2019). The elicitor acts as an effector and the optimal concentration depends on the presence of receptors, such as receptorlike kinases and wall-associated kinases, in the plant cell membrane. The interaction between the elicitor and the receptor occurs when the secondary metabolite reaches its optimal level (Pusztahelyi et al., 2015; Shalaby and Horwitz, 2015).
In this study, sucrose served as an elicitor to induce quercitrin production in C. morifolium callus. Each elicitor has an optimal concentration (Swaroopa and Anuradha, 2013), and excessively high concentrations can trigger a hypersensitive response that leads to cell death (Patel and Krishnamurthy, 2013). Sucrose acts as an osmotic agent, creating osmotic pressure that stimulates the generation of reactive oxygen species, such as hydrogen peroxide (H2O2), hydroxyl radicals (xOH), superoxide radicals (O2x-), and alkoxyl radicals (Rox). The production of H2O2 in the root suspension culture of Hypericum perforatum L. indicates oxidative stress, which serves as a second messenger in plant defense reactions (Cui et al., 2010). Thus, the increase in secondary metabolite biosynthesis is a result of the plant cells’ defense response (Wang et al., 2015). Sucrose activates various enzymes involved in flavonoid biosynthesis through the shikimate pathway, including PAL, chalcone synthase, chalcone isomerase, and isoflavone synthase (Liu et al., 2021; Lv et al., 2022). Although the pathway for quercitrin production during callus induction requires further investigation, this study demonstrates that sucrose can act as an elicitor for quercitrin production.
Previous studies have demonstrated that the production of quercetin can be enhanced by adjusting the composition of plant growth hormones in the cell culture media of Astragalus missouriensis and by supplementing the cell culture media of Citrullus colocynthis with phenylalanine. Quercetin production in these two cell cultures reached 8.1 and 7.3 mg/g DW, respectively, for A. missouriensis and C. colocynthis (Ionkova, 2009; Meena et al., 2014). Although the production of quercetin in chrysanthemum callus culture is not high as that of A. missouriensis and C. colocynthis cell cultures, the results of this study reveal the potential benefits of sucrose, which can act as an elicitor to increase quercetin content by up to 63.67%. In addition, this study provides new information regarding the benefits of sucrose as an alternative elicitor that is cheap and easy to apply to in vitro culture media.
Conclusion
The addition of sucrose to the culture media during callus induction led to an increase in quercitrin production. The extent of quercetin content increase varied between the 15th and 30th DAP harvest times. At the 15th DAP, the highest quercetin content (0.316 mg/g DW) in chrysanthemum callus culture was obtained in the 60 g/l sucrose treatment, representing a 27.94% increase. At the 30th DAP, the highest quercetin content (0.437 mg/g DW) was obtained in the 45 g/l sucrose treatment, showing a significant increase of 63.67%. Furthermore, all sucrose concentration treatments exhibited higher quercetin content compared to their respective content at the 15th DAP.
These results demonstrate that sucrose can act as an elicitor to increase the production of secondary metabolites like quercetin through callus culture. The potential of sucrose to stimulate the production of other secondary metabolites in different in vitro plant cultures is intriguing and warrants further investigation.