Introduction
Cirrhosis results from different mechanisms of liver injury including virus, alcohol, and non-alcoholic liver disease, which lead to necroinflammation and fibrogenesis [1]. Cirrhosis is the 14th most common cause of death in adults worldwide, and it results in 1.03 million deaths per year worldwide [2], which varies from 1% to 57% depending on the occurrence of clinical events [3]. Portal hypertension, ascites, spontaneous peritonitis, and encephalopathy are the most common and serious complications. Spontaneous peritonitis increases mortality in cirrhosis four times and has a poor prognosis [4]. A previous study has reported that vitamin D was closely related to spontaneous peritonitis [5].
Apart from classical effects on bone mineralisation, vitamin D also has distinct immunological functions influencing cell proliferation and differentiation, immunomodulation, and the gut microbiome [6]. In addition, another report indicated that the biologically active form of vitamin D, 1,25(OH)2D, may reduce inflammation [7].
The anti-inflammatory effect of vitamin D may be beneficial for the prognosis of spontaneous peritonitis. In this study, we aimed to conduct a meta-analysis to confirm whether there is a negative correlation between vitamin D and spontaneous peritonitis.
Material and methods
Data sources
Articles published up to 1 October 2019 in the PubMed, Medline, and Embase databases with restrictions of English-language medical literature for human studies were searched. Moreover, the following terms were used: ((vitamin D) OR (25-Hydroxyvitamin D) OR (calcitriol) OR VD (MeSH)), AND ((spontaneous peritonitis) OR (spontaneous bacterial peritonitis) OR (Peritonitis, Cirrhosis)).
Study selection
The articles must meet the following inclusion criteria and exclusion criteria. Inclusion criteria: 1) Human-related clinical research and full-text article. 2) Cirrhosis with spontaneous peritonitis. 3) complete data for the calculation of vitamin D. Exclusion criteria: 1) Duplicate article. 2) Similar article published by the same author and unit. 3) Published data were not accurate.
Data abstract
Risk of bias and quality assessment were assessed through elements of the STROBE checklist for studies included [8]. The work was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [9]. Two investigators independently examined included papers and abstract data in our analyses. If there was a disagreement, a third author would evaluate the disagreement again and form a final result after the trade. First of all, 25(OH)D was the main form of vitamin D considered in the included studies. Data were extracted to Microsoft Excel (2017 edition; Microsoft) for effective organisation. The following information was abstracted for analysis: basic characteristics (author, publication year, research country, and individuals of spontaneous peritonitis), serum 25(OH)D levels as continuous variables, and the cut-off level used to define 25(OH)D deficiency as a dichotomous variable, and so on. In addition, the cut-off level for 25(OH)D deficiency was defined as less than 20 ng/ml [10].
Statistical analysis
Odds ratio (OR) and 95% confidence intervals (CIs) were used to describe the ratio of the spontaneous peritonitis occurring in 25(OH)D deficiency individuals vs. controls. Standardised mean difference (SMD) was used for studies that reported mean and standard deviation (SD) values for 25(OH)D levels of spontaneous peritonitis individuals and controls. After the data were archived, pooled estimates were obtained using the random model (M-H heterology) method (I2 > 50%, p ≤ 0.1) [11]. Statistical heterogeneity was assessed by Cochran’s Q test and I2 statistic. In addition, sensitivity analysis was used to evaluate whether the results were stable and reliable. All analyses were carried out by metan in STATA 15.1.
Results
Basic characteristics
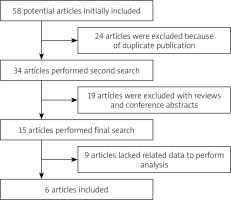
Our study initially identified 58 related references, of which six papers met our inclusion criteria [12–17]. The flowchart describing the process of study selection is shown in Figure 1. Five papers contained data describing mean ± SD of vitamin D in spontaneous peritonitis patients, while four papers described dichotomous data in spontaneous peritonitis patients. In addition, the unit of 25(OH)D using nmol/l was uniformly converted to ng/ml (1 nmol/l = 2.5 ng/ml). 25(OH)D was tested by enzyme-linked immunosorbent assay (ELISA) in five papers, and one paper chose chemiluminescence immunoassay.
Vitamin D deficiency and spontaneous peritonitis
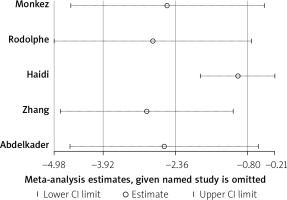
Five studies reported the 25(OH)D levels of spontaneous peritonitis patients and control individuals. The main characteristics are listed in Table I. The result showed that the average 25(OH)D level in spontaneous peritonitis patients was 2.36 less than that in control individuals (SMD = –2.36, 95% CI: –3.92, –0.8, I2 = 97.2%, p < 0.01; Figure 2). It showed significant heterogeneity. By sensitivity analysis, data from Haidi’s study made the result fluctuant. However, it was still stable and reliable (Figure 3). In addition, we aimed to determine whether individuals with vitamin D deficiency were more susceptible to spontaneous peritonitis. We integrated data from four articles (Table II), and the result showed that spontaneous peritonitis patients were 4.33 times more likely to be vitamin D deficient than controls (OR = 4.33, 95% CI: 1.57, 11.93, I2 = 50.1%, p = 0.111; Figure 4).
Figure 2
The average 25(OH)D level in spontaneous peritonitis patients was 2.36 less than that in control individuals (SMD = –2.36, 95% CI: –3.92, –0.8, I2 = 97.2%, p < 0.01)
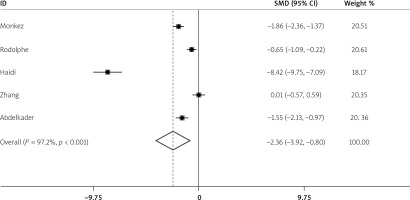
Figure 4
Spontaneous peritonitis patients were 4.33 times more likely to be vitamin D deficient than controls (OR = 4.33, 95% CI: 1.57–11.93, I2 = 50.1%, p = 0.111)
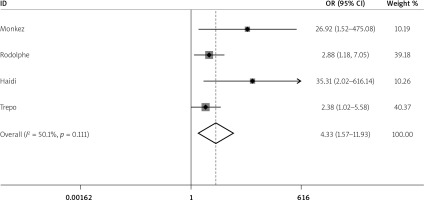
Table I
Basic characteristics of vitamin D in spontaneous peritonitis patients and controls
Discussion
There are few clinical studies on the relationship between vitamin D and spontaneous peritonitis. In this study, we collected relevant articles and data to derive the results, which demonstrated that cirrhosis patients with spontaneous peritonitis had lower vitamin D levels. And vitamin D deficiency had higher incidence rate of spontaneous peritonitis. It can be seen that vitamin D may be an important protective factor in cirrhosis patients with spontaneous peritonitis.
Vitamin D is involved in a variety of biological processes. Vitamin D exerts its biological activity via combination with VDR, which expresses in almost all target tissues,. The mechanisms of increased risk of infection in cirrhotic patients with a low 25(OH)D level are still unclear. The most likely mechanism is the anti-inflammatory effect of vitamin D. As is well-known, vitamin D is mediated by antigen-presenting cells such as macrophages and dendritic cells. The active form of the 1,25-OH vitamin D binding to vitamin D receptor, which is able to increase the production of proteins with antibacterial effects such as LL-37 cathelicidin, a vitamin D-dependent endogenous antibacterial peptide [18, 19]. LL-37 is closely related to infection. Liu et al. showed that lower LL-37 and vitamin D-mediated innate immunity may contribute to susceptibility to microbial infection [20]. Other studies also reported the role of LL-37 in Streptococcus suis and Staphylococcus aureus [21, 22]. Xie et al. found that Streptococcus suis blunts the innate host defences via ApdS protease-mediated LL-37 cleavage [22]. Also, Kang et al. revealed that LL-37 has potential clinical application in the eradication of biofilms and treatment of prosthetic joint infection [21]. In addition, in patients undergoing haemodialysis, lower LL-37 led to a higher risk of death from infection [23]. In a follow-up study conducted by Zhang et al. it was reported that vitamin D supplementation could up-regulate peritoneal macrophage VDR and LL-37 expressions, which resulted in an enhanced immunological defence against spontaneous peritonitis in patients with cirrhosis and ascites [24]. The mechanism may be that in the ascitic fluid in spontaneous peritonitis patients, decreased secretion of Th2-type cytokines such as IL-4, IL-10, and IL-13 induce Bcl-3, which may result in the change in LL-37 regulation [15].
Another potential mechanism might be linked to the gut microbiota, which recently emerged as an important actor implicated in chronic hepatic disorders through the gut–liver axis [25]. Gut bacterial overgrowth and increased gut permeability could lead to an increased risk of bacterial translocation in the enterocoelia. On the one hand, vitamin D itself inhibits cirrhosis progression. Roth et al. reported that rats fed a vitamin-deficient diet had significantly worsened steatosis and more lobular inflammation than controls [26]. On the other hand, Liu et al. proved that vitamin D can protect the gut mucosal epithelial barrier and strengthen the tight junction, which could inhibit bacterial translocation [27].
Although we have achieved encouraging results, there were still some shortcomings. The most important issue was the heterogeneity of included articles. Although the sensitivity analysis showed that the results was still stable, some factors still may lead to the existence of heterogeneity. First, differences in vitamin D detection methods, types and causes of cirrhosis, and the number of patients enrolled may be sources of heterogeneity. The emergence of heterogeneity might reduce the credibility of the conclusion, while it could still provide sufficient evidence. Second, there were too few articles to provide data of analysis. We believe that a greater number of articles can provide more data and research evidence. In addition, this meta-analysis lacked prospective randomised controlled studies. Therefore, we could not directly confirm the causal relationship between vitamin D and spontaneous peritonitis. Infection can lead to decreased absorption of the gastrointestinal tract, which may affect vitamin D absorption. These are points that follow-up research can improve.
Conclusions
This meta-analysis demonstrated that vitamin D levels in cirrhosis patients with spontaneous peritonitis were lower than controls, suggesting that vitamin D may be a protective factor in spontaneous peritonitis. In the future, more prospective studies are needed to validate the results of this study.