INTRODUCTION
Reactive oxygen species (ROS) encompasses various oxygen-containing free radicals, including superoxide anion (
Living an active lifestyle that includes moderate exercise has been demonstrated to prevent various diseases and improve antioxidant defenses [7]. Under normal exercise training, ROS levels do not reach detrimental levels due to exercise-induced fatigue. However, during strenuous exercise training, ROS can reach a detrimental level, a phenomenon proposed as hormesis [5]. Intense exercise produces high levels of ROS that cannot be entirely counteracted by the antioxidant system, and the high levels of ROS may impair cellular function and even limit exercise performance [8]. During intense exercise, several major factors contribute to the production of high levels of ROS, including ischemia/reperfusion, increased mitochondrial activity, and the activation of enzymes in the muscles [5]. A study reported that exercising at an intensity exceeding 50% of
Vitamin D, an essential vitamin, exists in two forms, namely vitamin D2 and vitamin D3. Vitamin D2 is mostly present in plants, yeast, and fungi, whereas vitamin D3 is found in animal-based foods such as salmon, tuna, sardine, and liver [11]. Alternatively, the human body can produce vitamin D upon exposure to ultraviolet light (UV) in the band B range, which is why vitamin D is commonly referred to as the sunshine vitamin [12]. The active form of vitamin D, 1,25(OH)2D, regulates a series of physiological functions, including the immune system, bone health, cardiac function, muscle damage, and oxidative stress [6, 13, 14].
Vitamin D3 was first identified as a potential antioxidant by Wise-man in 1993, who suggested that the hydrophobic portions of 1,25(OH)2D3 can reduce membrane viscosity, thereby protecting the cell membrane from free radicals and lipid peroxidation [15]. In addition, vitamin D deficiency has been reported to cause mitochondrial dysfunction, leading to ROS production [16]. Studies have also demonstrated that vitamin D deficiency promotes lipid and protein oxidation in the skeletal muscle and alters antioxidant enzyme activity [17, 18]. Ke et al. randomized rats into four groups: a control group, a vitamin D3 supplementation group, an exercise intervention group, and a group subjected to both vitamin D3 supplementation and exercise intervention. After the rats in the exercise intervention groups completed a high-intensity exercise intervention, all rats were injected with normal saline or vitamin D3 (1 ng/mL). Compared with the rats in the exercise-only group, those in the group subjected to vitamin D3 supplementation combined with the exercise intervention exhibited significantly decreased concentrations of the oxidative product 4-hydroxynonenal in their kidneys and lungs [19]. In another study, healthy men received 3,600 IUs of daily vitamin D3 supplementation for two weeks; thereafter, their oxidative stress and antioxidant indices during elastic-band resistance training were measured; the results indicated that vitamin D3 supplementation did not significantly alter oxidative stress markers and antioxidant enzyme concentrations [20].
Studies examining the effects of vitamin D3 supplementation on oxidative stress have reported inconsistent results, with some indicating a reduction in oxidative stress and others failing to identify any significant effects. In addition, the clinical translation of these in vitro and in vivo results remains unclear. To address these questions, we conducted a trial to evaluate the effects of 5,000 IUs of daily vitamin D3 supplementation over four weeks on strenuous endurance exercise (SEE)-induced oxidative stress.
MATERIALS AND METHODS
Participants
The statistical power analysis software program, G*Power (v. 3.1.9.7), was used to calculate the sample size in the present study. With a 2 × 4 two-way mixed-design analysis of variance (ANOVA), an effect size of 0.4, an α value of 0.05, and a power of 0.95, the appropriate sample size was determined to be 16. Therefore, our study recruited 26 healthy men without any underlying diseases (e.g., cardiovascular disease, diabetes, kidney disease, liver disease, and autoimmune disease). Sex differences are apparent in oxidative stress response and vitamin D concentrations. Therefore, we opted to include only male participants to minimize variation in the study [21, 22]. Young Taiwanese males aged between 20 and 40 were invited to participate in the study through word-of-mouth invitations at Taipei Medical University. Individuals with acute sports injuries, a blood 25(OH)D concentration of > 30 ng/mL, and a
Experimental design
For the present study, a double-blinded matched-pair design was adopted, and 26 participants were divided into a placebo (n = 13) group and a vitamin D3 (n = 13) group on the basis of their
Supplementation
While a daily dose of 4,000 IU of vitamin D is considered the tolerable upper intake level for adults in some authorities, the Endocrine Society recommends treating adults with vitamin D deficiency (< 30 ng/mL) with 50,000 IU of vitamin D2 or D3 once a week for eight weeks to achieve a blood level of 25(OH)D above 30 ng/mL [23]. Besides, the dose of 5,000 IU of vitamin D is half the upper limit of the recommended intake for adults (19–70 years) according to the Endocrine Society. Additionally, research suggests that the body utilizes an average of 3,000–5,000 IU of vitamin D daily [24]. Furthermore, the population we are specifically targeting vitamin D-deficient athletes who participate in strenuous endurance exercise, which may warrant different supplementation recommendations. Therefore, the supplementation dosage and method used in this study were consistent with those employed in another study that successfully raised vitamin D levels from insufficient to sufficient while considering safety [25]. In the present experiment, the individuals in the supplementation group consumed 5,000 IUs of liquid-form vitamin D3 (Liquid Shield Vitamin D3+E, Panion & BF Biotech, Taipei, Taiwan) daily after lunch for four weeks. By contrast, the placebo group consumed medium-chain triglycerides with the same color, taste, and odor as the vitamin D3 supplement (Panion & BF Biotech). Through regular messages, all participants were reminded to take the supplement capsules as prescribed and were monitored to ensure that the vitamin D supplementation did not lead to any adverse effects. Compliance was assessed by calculating the number of leftover capsules.
Blood sampling and analysis
Before and after the vitamin D3 intervention, 1 mL of blood sample was drawn from the antecubital vein of each participant and centrifuged at 3,000 rpm for 10 min at 4°C (Centrifuge 5702 R; Eppendorf; Hamburg, Germany). To measure the 25(OH)D concentration, the serum obtained after centrifugation was collected and tested on an automated immunoassay analyzer (Cobas e801; Roche Diagnostics, Mannheim, Germany) at the Taipei Medical University Hospital.
From the participants, additional blood samples (1 mL) were drawn from the antecubital vein and collected in ethylenediaminetetraacetic acid tubes before (pre-SEE), immediately after (post-0), two h after (post-2), and 24 h after (post-24) the SEE test. The ethylenediami-netetraacetic acid tubes were centrifuged at 3,000 rpm for 10 min at 4°C (Eppendorf Centrifuge 5702 R), and the supernatant was collected in microcentrifuge tubes, stored at −80°C, and subsequently analyzed for TBARS, PC, GPX, SOD, and CAT concentrations.
The TBARS concentration was measured using colorimetric methods. Specifically, 200 μL of plasma or the 1,1,3,3-tetramethoxypropane standard was mixed with 1 mL of the TBARS reagent (15% trichloroacetic acid and 0.38% thiobarbituric acid in 0.25 N HCl). The tubes were then placed in a dry bath set to a temperature of 100°C for 30 min. Thereafter, the tubes were cooled on ice for 10 min and centrifuged (3,900 rpm) at room temperature for 10 min. Next, 200 μL of the supernatant was transferred to microplate wells, and absorbance was read at 535 nm. PC were quantified using a commercial enzyme-linked immunoassay protein carbonylation kit (Cat. no ab238536; Abcam, Cambridge, MA, USA) as per the manufacturer’s instructions. SOD analysis was performed using a SOD determination kit (Cat. no 19160; Sigma-Aldrich, St. Louis, MO, USA) as per the manufacturer’s instructions. In addition, CAT and GPx concentrations were also measured using an assay kit (Cat. no 707002; Cat. no 703102; Cayman, Ann Arbor, MI, USA) as per the manufacturer’s instructions.
Graded exercise test (GXT) protocol
The participants completed a GXT to establish a reference for determining the intensity of the SEE test (65%
Strenuous endurance exercise (SEE) test
The participants underwent the SEE test 2 days after completing the GXT test. After arriving at the laboratory, and rested for 30 min and wore a heart rate monitor (S610; Polar, Kempele, Finland) for subsequent heart rate monitoring during the SEE test. After performing a 5-min warm-up routine (heart rate ≤ 150 beats/min), each participant cycled on a cycle ergometer at 65% of
Statistical analysis
The normality of data distribution was evaluated using the Shapiro–Wilk test. As some parameters were not normally distributed, non-parametric methods were employed, and median and interquartile range (IQR) were used to present all data. Participant characteristics and serum 25(OH)D concentrations were analyzed using the Mann–Whitney U test and Wilcoxon signed-rank test. The significance of differences between the vitamin D3 group and the placebo group for other variables was analyzed using the Mann–Whitney U test. Different time points were statistically compared using the Friedman test and Wilcoxon signed-rank test as a post hoc test. Statistical significance was set at p < 0.05. Cohen’s d values were used to estimate effect sizes, which were categorized as small (0.20–0.49), moderate (0.50–0.79), and large (≥ 0.80). When p < 0.05, the data sets were regarded as statistically significant. All statistical analyses were performed using SPSS version 22.0 (IBM, Armonk, NY, USA).
RESULTS
Effects of vitamin D3 supplementation on serum 25(OH)D concentration
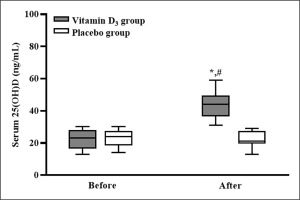
After counting the leftover capsules, the adherence to supplementation was 100%. The serum 25(OH)D concentrations of the participants in the placebo and vitamin D3 groups before and after vitamin D3 supplementation are listed in Figure 2. In the placebo group, the participants’ serum 25(OH)D concentrations before and after supplementation were not significantly different (p > 0.05). In the vitamin D3 group, the participants’ serum 25(OH)D concentration was significantly higher after supplementation than before supplementation (p < 0.05), and the serum 25(OH)D concentration of the vitamin D3 group was significantly higher than that of the placebo group (p < 0.05; d = 3.12). Our results indicated that four weeks of vitamin D3 supplementation effectively increased the serum 25(OH) D concentration.
Effects of vitamin D3 supplementation on oxidative damage
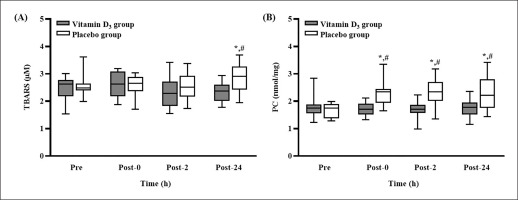
The TBARS and PC concentrations after 4 weeks of vitamin D3 or placebo supplementation are presented in Figure 3. The TBARS concentration in the placebo group was significantly higher at post-24 than at pre-SEE (p < 0.05); at post-24, it was significantly higher than that in the vitamin D3 group (p < 0.05, d = 1.03) (Figure 3A). In addition, the concentration of PC in the placebo group was significantly higher at post-0, post-2, and post-24 than at pre-SEE (all p < 0.05); it was also significantly higher than that in the vitamin D3 group at the three post-SEE time points (all p < 0.05; d = 1.06 [post-0], 1.00 [post-2], and 0.91 [post-24]) (Figure 3B).
FIG. 3
Changes in (A) TBARS and (B) PC concentrations after strenuous endurance exercise. * p < 0.05 compared with baseline concentration. # p < 0.05 compared with concentration in vitamin D3 group. Abbreviations: TBARS, thiobarbituric acid reactive substances; PC, protein carbonylation; Pre-SEE, before exercise; Post-0, immediately after exercise; Post-2, 2 h after exercise; Post-24, 24 h after exercise.
Effects of vitamin D3 supplementation on antioxidant enzyme concentrations
The levels of SOD, CAT, and GPx activity after 4-week vitamin D3 or placebo supplementation are presented in Figure 4. In the placebo group, the level of SOD activity was significantly higher at post-0, post-2, and post-24 than at pre-SEE (all p < 0.05). Moreover, the level of SOD activity in the placebo group was significantly higher than that in the vitamin D3 group at the three post-SEE time points (all p < 0.05; d = 1.13 [post-0], 1.00 [post-2], and 1.19 [post-24]) (Figure 4A). In the placebo group, the level of CAT activity was significantly higher at post-0 than at pre-SEE (p < 0.05). Moreover, the level of CAT activity in the placebo group was significantly higher than that in the vitamin D3 group at post-0 (p < 0.05; d = 0.94) (Figure 4B). Nevertheless, the level of GPx activity in the placebo group was significantly higher at post-0 than at pre-SEE (p < 0.05) (Figure 4C).
FIG. 4
Changes in (A) SOD, (B) CAT, and (C) GPx concentrations after strenuous endurance exercise. * p < 0.05 compared with baseline concentration. # p < 0.05 compared with concentration in vitamin D3 group. Abbreviations: SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; Pre-SEE, before exercise; Post-0, immediately after exercise; Post-2, 2 h after exercise; Post-24, 24 h after exercise.

DISCUSSION
According to the US Endocrine Society, vitamin D insufficiency is defined as a 25(OH)D of 21–29 ng/ml [23]. After four weeks of 5,000-IU daily vitamin D3 supplementation, the serum 25(OH)D concentration of the participants in the vitamin D3 group significantly increased from an insufficient level (median: 23 ng/mL) to a sufficient level (median: 44 ng/mL). This result is consistent with other studies, which reported that four weeks of 5,000-IU daily vitamin D3 supplementation effectively increased serum 25(OH)D concentrations to mitigate vitamin D deficiency [14, 25]. Thus, four weeks of 5,000-IU daily vitamin D3 supplementation is an appropriate strategy for increasing the serum 25(OH)D concentration in vitamin D insufficient (< 30 ng/mL) athletes.
In the placebo group, the concentration of TBARS was significantly elevated at post-24, and the concentration of PC was significantly elevated at post-0, post-2, and post-24. These results indicate that the SEE protocol induced significant oxidative damage. Studies have reported that SEE significantly increased the concentrations of TBARS and PC [10, 13]. However, in the present study, the concentration of TBARS was not significantly elevated at post-0 and post-2. In a study that examined exercise-induced muscle damage, the blood concentration of TBARS peaked at post-24 in the placebo group [26]. Additionally, research has demonstrated that aerobic exercise increases the likelihood of protein oxidation and carbonylation formation. By contrast, rigorous isometric exercise increases the likelihood of lipid peroxidation [27]. These findings may explain why only the concentration of PC and not that of TBARS was significantly elevated at post-0 and post-2 in the present study. Furthermore, our laboratory indicated that our intense exercise protocol induced muscle damage [14]. PC have also been demonstrated to exhibit the highest correlation coefficients with muscle damage, and this association also contributes to the discrepancy [28]. Additionally, in the present study, the concentration of PC in the placebo group was significantly higher than that in the vitamin D3 group at post-0, post-2, and post-24. The concentration of TBARS in the placebo group was significantly higher than that in the vitamin D3 group at post-24, suggesting that vitamin D3 supplementation attenuated oxidative damage. Studies have demonstrated that an increased blood 25(OH)D concentration can significantly reduce the levels of various markers of oxidative damage under specific conditions (e.g., vitamin D–deficient diet, low back pain, and polycystic ovary syndrome) [17, 18, 29]. Furthermore, given that excessive levels of ROS cause secondary muscle damage [30], our results correspond to those of another study, which suggested that vitamin D3 supplementation reduces ROS production, leading to reduced SEE-induced muscle damage [14]. The present study is the first human study to report that vitamin D3 supplementation can significantly prevent SEE-induced oxidative damage. Therefore, four weeks of 5,000-IU daily vitamin D3 supplementation is an appropriate strategy for alleviating SEE-induced oxidative damage in vitamin D insufficient (< 30 ng/mL) athletes.
In the present study, the level of SOD activity in the placebo group was significantly higher at post-0, post-2, and post-24 than at pre-SEE. In the same group, the levels of CAT and GPx activity were significantly higher at post-0 than at pre-SEE. The results for the markers of oxidative damage indicated a significant increase in the antioxidant system activity in the placebo group with oxidative damage but not in the vitamin D3 group without oxidative damage. In the present study, antioxidant enzymes were activated only when oxidative damage occurred, which aligns with the findings of another study [31]. Similarly, a study reported that intense exercise increased the activity of antioxidant enzymes, which counteracted oxidative stress [32]. Alternatively, a study suggested that vitamin D3 exhibits antioxidant properties that can mitigate oxidative damage. These properties can be attributed to several mechanisms. First, a previous study demonstrated that vitamin D contains hydrophobic parts that can stabilize and protect the membrane from lipid peroxidation [15]. Additionally, an in vitro study conducted in 2005 suggested that vitamin D3 exerts a stronger antioxidant effect on zinc-induced oxidative stress than vitamin E, β-estradiol, and melatonin [33]. Second, researchers have argued that vitamin D reduces the gene expression of NADPH oxidase, which is the primary generator of ROS [34]. Finally, a study indicated that vitamin D mediates ROS production through the Nrf2-ARE-signaling pathway by increasing the activity of nuclear factor-erythroid factor 2-related factor 2, which is a transcription factor that controls the basal and induced expression of a series of antioxidant response elements [35]. Therefore, our results suggest that vitamin D3 supplementation is adequate for managing SEE-induced oxidative stress, possibly because of its inherent antioxidant capacity. However, the exact mechanism by which vitamin D3 mediates oxidative stress is still unclear. Additional research is required to clarify the molecular mechanisms underlying the effects of vitamin D3 supplementation on SEE-induced oxidative stress.
At present, the in vivo findings regarding the antioxidant effects of vitamin D on skeletal muscle are limited. In a study by Dzik et al., patients with both low back pain and vitamin D deficiency consumed 3,200 IUs of vitamin D for five weeks, and they experienced significant decreases in the concentrations of 8-isoprostanes and PC in their paraspinal muscle along with significant decreases in cytosolic SOD and GPx activity; these finding [18] are similar to those of our study. However, in other studies examining either rats or human participants with other diseases, the opposite effect was reported, suggesting that vitamin D3 supplementation increases antioxidant activity [17, 36]. The differences between the findings of these studies may be due to species differences (humans vs. rats), muscle pathology, or differences in the sources of oxidative damage. As was discussed in an earlier part of the present study, we assumed that vitamin D deficiency increased oxidative stress, thereby leading to increased SOD, CAT, and GPx activity; furthermore, vitamin D3 supplementation improved oxidative damage and the balance of the antioxidant system. Nevertheless, human studies should be conducted to further clarify the effects of vitamin D on the antioxidant status and the related mechanisms.
Maintaining an adequate concentration of serum 25(OH)D is essential for athletes. A study recommended a blood 25(OH)D concentration of > 40 ng/mL for athletes because this is the threshold for the onset of vitamin D storage in muscles and fat [24]. Under such circumstances, liver hydroxylation shifts to zero-order kinetics, and tissue calcitriol levels depend on skin synthesis or oral intake below this range, indicating substrate starvation [37]. Similarly, we demonstrated that serum 25(OH)D concentrations of ≥ 42 ng/mL had a positive effect on SEE-induced oxidative damage immediately after exercise. However, our previous study revealed that serum 25(OH)D concentrations of ≥ 44 ng/mL did not affect the time to exhaustion and
The strength of this study lies in its status as the first human study to explore the effects of relatively higher vitamin D3 supplementation doses on oxidative stress and antioxidant enzymes, demonstrating that vitamin D supplementation can prevent exercise-induced oxidative damage. However, the study has limitations, including a lack of assessment for indicators of adverse effects such as hypercalcemia. Previous studies have shown that calcium levels remain normal with a daily dose of 8,000 IU after 12 weeks of supplementation, with similar participant characteristics [39]. Additionally, the study did not include fat mass data in the participant characteristics and had a limitation of lower BMI, which may have minor effects on vitamin D metabolism. Nevertheless, there were no significant BMI differences between the two groups, suggesting similar anthropometric conditions to some extent.
CONCLUSIONS
Our results indicate that 5,000 IUs of oral vitamin D3 supplementation (oil form) for 4 weeks effectively increases the serum 25(OH)D concentration to prevent oxidative damage (TBARS and PC). Our study preliminarily supports the implementation of a nutritional supplementation strategy that can mitigate SEE-induced oxidative damage in athletes performing intensive training. Therefore, future studies should investigate the potential cellular mechanism by which vitamin D3 reduces SEE-induced oxidative damage as well as the effects of vitamin D3 supplementation at various dosages or over various periods.