Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease, which is characterized by recurrent eczema and intense itching. It has a great impact on the mental health of patients and accounts for the major burden of global skin diseases. The aetiology of AD covers a multitude of factors, like the impaired skin barrier function, unbalanced immunity, genetic susceptibility and environmental exposure. There is no cure for AD yet. Corticosteroids, as the main treatment for AD, is still limited by adverse side effects, poor compliance and recurrence after drug withdrawal [1].
Lately, the JAK-STAT pathway has been identified to have a major role in the aetiology of AD. The clinical trials have shown that upadacitinib, an oral JAK1-selective inhibitor, is effective and well tolerated for moderate-to-severe AD [2]. Additionally, upadacitinib has been approved by the European Drug Administration and the US Food and Drug Administration for patients with moderate-to-severe AD.
Although the drug shows great potential, there is no meta-analysis to summarize and quantify the efficacy and safety of the drug at present.
Aim
Therefore, this meta-analysis aims to evaluate the overall efficacy and safety of the drug in adults and children with AD.
Material and methods
Search strategy
The present study was performed according to PRISMA guidelines [3]. Electronic searching was conducted using PubMed, Embase and the Cochrane Library between inception date and January 2023. We combined the terms: “atopic dermatitis” AND “upadacitinib” as either key words or MeSH terms.
Selection criteria
Eligible studies for the meta-analysis included those in which patients from randomized controlled trials (RCTs) with AD were treated with upadacitinib and placebo therapy. The studies must be published in English. Studies were excluded according to the title/abstract or full text. Those studies without reported efficacy and safety outcomes were excluded. Some abstracts, conference presentations, editorials, reviews and expert opinions were also excluded.
Data extraction
Two researchers respectively extracted data from included studies and settled the disagreements through discussion. They carefully reviewed each study and collected the following data: lead author, publication year, the number of cases and controls, median age, sex ratio, treatment regimen, background therapy, and treatment duration. And certain efficacy and safety outcomes were extracted as the endpoints.
For efficacy outcomes, the percentage of participants achieving at least a 75% reduction in Eczema Area and Severity Index score (EASI 75) and a reduction of ≥ 4 points in worst pruritus Numerical Rating Scale from baseline (NRS4) at week 16 were recorded; for safety outcomes, serious adverse events (AEs) and some common AEs (including nasopharyngitis, upper respiratory tract infection, blood creatine phosphokinase increase, headache and acne) were recorded.
Assessment of risk of bias
The quality of the included studies was assessed by two authors using the Cochrane collaboration’s risk-of-bias tool. The items listed below were evaluated and recorded: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The risk of bias for each included study was assessed at “low risk”, “some concern”, or “high risk”. Sensitivity analysis was conducted to evaluate the stability of the results. Regarding the small number of included studies, we did not assess potential publication bias.
Statistical analysis
For RCTs, analyses were performed using Stata 17.0. If the P value of Cochrane Q statistics was less than 0.05 or I2 statistics was greater than 50%, it means that the included studies had significant heterogeneity. Then, we chose the random effects meta-analysis model, otherwise we chose the fixed effect model. The overall pooled RR with 95% CI was presented as the primary result.
Results
Study selection
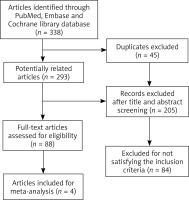
Overall, 338 records were identified through three databases. After removing 45 duplicates, we excluded 205 records based on the title and abstract. The remaining 88 publications were screened for full text. After a thorough review of these reports, 4 RCTs [4–6], recruiting 2,959 participants in total, were enrolled to this analysis (Figure 1).
Study characteristics and risk-of-bias assessment
We found 4 studies comparing the clinical outcomes between upadacitinib and placebo in moderate-to-severe AD patients. Among 4 studies, 3 RCTs [4, 6] were phase 3 and 1 RCT [5] was phase 2. In the study by Emma et al. [5], participants were randomized into 4 arms of upadacitinib 30 mg, 15 mg, 7.5 mg, and placebo. The other three studies [4, 6] were three-arm studies with upadacitinib 30 mg or 15 mg or placebo. Three studies [4, 6] recruited adults and adolescents respectively, while the remaining one [5] enrolled only adults. Ultimately, data were extracted for the 15 mg and 30 mg dose of upadacitinib and placebo in adolescents and adults, respectively. The treatment duration of all studies was 16 weeks. Characteristics of the enrolled studies were presented in Table 1.
Table 1
Characteristics of studies included in the meta-analysis
[i] QD – once daily, ① EASI-75 response: ≥ 75% reduction in Eczema Area and Severity Index score; ② NRS4 response: ≥ 4 points reduction in worst pruritus Numerical Rating Scale from baseline; ③ serious adverse events; ④ nasopharyngitis; ⑤ upper respiratory tract infection; ⑥ blood creatine phosphokinase increase; ⑦ headache; ⑧ acne.
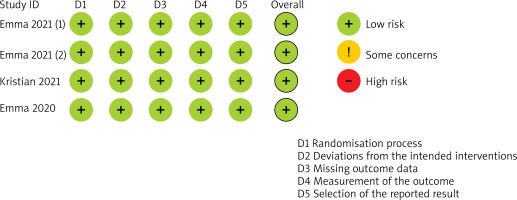
By excluding the included studies one by one, we observed that the sensitivity analysis result was stable. Overall, the included RCTs were recognized as being at low risk of bias. Risk-of-bias assessment of included studies was described in Figure 2.
Efficacy outcomes for adults
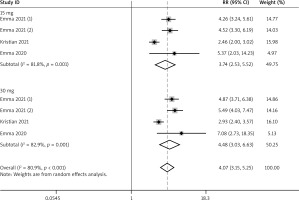
A total of 4 RCTs reported the efficacy outcomes. We adopted the random and fixed effect models respectively for EASI-75 and NRS4 at week 16 (I2 = 80.9%; I2 = 29.3%). For included efficacy outcomes, adults treated with upadacitinib all had better performance than controls (EASI-75: RR = 4.68, 95% CI: 4.09, 5.35; NRS4: RR = 4.07, 95% CI: 3.15, 5.25) (Figures 3 and 4). In the sub-group analysis, the fixed effect model analysis revealed that upadacitinib 30 mg was better than 15 mg (EASI-75: RR = 1.19 (1.12, 1.26), p < 0.05; NRS4: RR = 1.24 (1.14, 1.35), p < 0.05) (Table 2).
Table 2
Upadacitinib of 30 mg vs. 15 mg for efficacy outcomes
| Age group | No. of patients (No. of trials) | EASI-75 (30 mg vs. 15 mg) | NRS4 (30 mg vs. 15 mg) | ||
|---|---|---|---|---|---|
| RR (95% CI) | I2 (%) | RR (95% CI) | I2 (%) | ||
| Adults | 2,365 (4) | 1.18 (1.11, 1.25)* | 0 | 1.24 (1.14, 1.35)* | 49.70% |
| Adolescents | 552 (3) | 1.15 (1.02, 1.30)* | 29.40% | 1.25 (1.01, 1.54)* | 0 |
Efficacy outcomes for adolescents
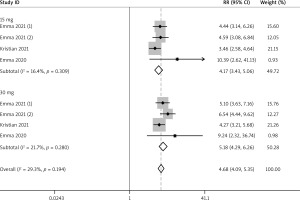
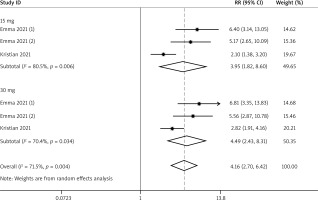
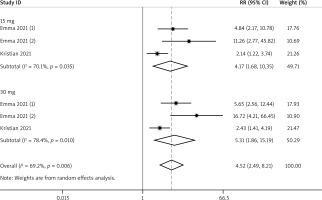
A total of 3 RCTs reported the efficacy outcomes. And the random effects models were used for both EASI-75 and NRS4 at week 16 (I2 = 71.5%; I2 = 69.2%). At week 16, more adolescents in the upadacitinib group achieved EASI-75 and NRS4 response than controls (EASI-75: RR = 4.16, 95% CI: 2.70, 6.42; NRS4: RR = 4.52, 95% CI: 2.49, 8.21) (Figures 5 and 6). In sub-group analysis, the fixed effect model analysis revealed that upadacitinib 30 mg was more effective than 15 mg for adolescents (EASI-75: RR = 1.15 (1.02, 1.30), p < 0.05; NRS4: RR = 1.25 (1.01, 1.54), p < 0.05) (Table 2).
Adverse events
For serious AEs and some common AEs (including nasopharyngitis, upper respiratory tract infection, blood creatine phosphokinase increase, headache and acne), they were all reported in four studies. The fixed effect models were adopted for all the included AEs (I2 = 0). For serious AEs, upper respiratory tract infection and headache, the results indicated that there was no significant difference between the group with upadacitinib and controls (RR = 0.74, 95% CI: 0.46, 1.20, p = 0.218; RR = 1.29, 95% CI: 0.97, 1.72, p = 0.082; RR = 1.41, 95% CI: 1.00, 2.00, p = 0.053). However, patients with upadacitinib are more prone to the following AEs: nasopharyngitis: RR = 1.35 (1.02, 1.78), p = 0.033; blood creatine phosphokinase increase: RR = 2.18 (1.38, 3.43), p = 0.001; acne: RR = 5.45 (3.55, 8.38), p < 0.001 (Table 3).
Table 3
Summary of adverse events of upadacitinib for moderate-to-severe AD
Discussion
The results showed that upadacitinib had good efficacy and safety in moderate-to-severe AD, and the real-world data also confirmed that. A cohort study analyzed 43 adults with AD. After treated with upadacitinib 30 mg daily for 16 weeks, 97.5%, 82.1% and 69.2% of the patients achieved EASI 75, EASI 90 and EASI 100, respectively. 16/43 patients reported AEs during the period, and most of the AEs were evaluated as mild [7]. Napolitano et al. enrolled 9 patients with AD. They all previously failed the treatment with dupilumab due to ineffectiveness or AEs. After enrolment, they received upadacitinib of 30 mg once a day (baseline: EASI 27.2 ±3.5, DLQI 24.8 ±4.1, NRS 8.9 ±0.9). At week 4 and 16, they all demonstrated significant improvements (week 4: EASI 7.3 ±2.9, DLQI 5.1 ±3.7, NRS 0.3 ±0.5; week 16: EASI 3.3 ±2.3, DLQI 3.9 ±2.2, NRS 0.2 ±0.4). Notably, by the first week, the NRS had already decreased by 79.77% compared to baseline (p < 0.0001). And 5 (55.57%) patients showed pruritus relief on day 2. This indicated that upadacitinib can quickly alleviate itching in AD. There were no AEs [8].
A recent review has demonstrated no significant correlation between the occurrence of AEs and the dose of upadacitinib [9]. In our results, most of the AEs were not serious, and no death was reported. It was revealed that there was a higher risk of nasopharyngitis, increased blood creatine phosphokinase and acne in the upadacitinib group. Among them, increased blood creatine phosphokinase mostly occurred after excessive exercise and had no clinical manifestations [10]. Acne was the most frequently reported AE, usually reported as mild-to-moderate. First, the epidemical investigation showed that AD was not associated with acne. Besides, acne is also known as a common adverse effect of other JAK inhibitors such as ruxolitinib and baricitinib. The above indicated that acne was most likely caused by upadacitinib in our study, but it is certainly noted that most people had used corticosteroids, which can also increase the incidence of acne [7].
Blauvelt et al. [11] recruited 692 adult patients, which were randomly assigned to oral upadacitinib (30 mg QD) or subcutaneous dupilumab (300 mg every other week). At week 4, about 70% of patients reached EASI 75 in the upadacitinib group, while only about 36% in the dupilumab group; at week 16, around 71% of patients reached EASI 75 in the upadacitinib group, while 61.1% in the dupilumab group. It revealed that upadacitinib is faster and more effective than dupilumab in treating moderate-to-severe AD, with no new safety risks.
In an open-label trial, 6 patients previously had failed a variety of treatments including dupilumab. After being treated with upadacitinib for 4 weeks, all patients reached EASI-75 [12]. Three patients with facial AD had failed dupilumab therapy, but they were successfully treated after switching to upadacitinib 15 mg bid [13]. The above suggested that upadacitinib can be used for resistant AD or facial AD. AD and alopecia areata (AA) have shared pathogenesis, such as the overexpression of helper T cell (Th) 2 cytokines interleukin-4 (IL-4) and IL-13. The JAK/STATS signal transduction pathway is a common potential target for both [14]. And there have been some cases of AD and AA patients successfully treated with upadacitinib [15]. Cantelli et al. reported a 24-year-old patient with severe AD and AA. After treatment with dupilumab, he showed no improvement in AA and developed a paradoxical “red face”. Then he switched to upadacitinib (30 mg/day). 3 months later, the patient showed significant improvement in both AD and AA, and no AEs occurred [14]. When managing patients with both AD and AA, upadacitinib may be a good option.
First, the number of included studies is small, especially for children. However, most of the included studies are large-scale and of high quality, and the real-world data also supported that upadacitinib had great potential for AD. Second, we did not set subgroups of dosage or age for AEs. However, there is no heterogeneity in included AEs (I2 = 0), indicating that the result has certain reliability.