Introduction
Atopic dermatitis (AD) is a chronic, recurrent, pruritic, non-infectious, inflammatory disease. The lesions, characterized by papules, patches, vesicles, and crusting, vary depending on age, race, sex, and geographic location. AD patients are more susceptible to other atopic diseases such as asthma, allergic rhinitis, and food allergies [1]. Recently, the incidence of AD has been increasing steadily for several decades, not only in developed countries, but also in developing countries [2]. The pathogenesis of AD is multifactorial, including immune dysregulation, gene mutation, impaired skin barrier function, and environmental factors [3].
Emollients and topical therapies such as low-potency topical steroids are used to control mild AD [4]. In contrast, topical therapies are inadequate for the control of severe AD. When topical therapies fail, patients with moderate-to-severe AD always require systemic immunosuppressants to control symptoms. However, long-term use of these immunosuppressants can cause severe adverse events such as liver and kidney damage [3, 5]. In recent years, targeted monoclonal antibodies have got attention wildly to treat moderate-to-severe AD for the potential to provide effective, safer treatment of uncontrolled AD. Dupilumab, an interleukin (IL)-4 receptor antagonist, was the first approved biological agent to treat AD and showed promising efficacy with acceptable safety [6, 7]. However, there are still some patients who cannot achieve satisfactory efficacy by using dupilumab [8, 9]. Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathway (JAK-STAT pathway) stands essential for the signalling of Th2 cytokines (IL-4, IL-13, IL-31), which play an essential role in the pathogenesis of AD [10]. JAK inhibitors, as a novel therapeutic approach, get people’s attention.
Upadacitinib, an oral selective-JAK1 inhibitor, was approved in 2019 to treat moderate-to-severe active rheumatoid arthritis and an inadequate response or intolerance to methotrexate [11]. Several 2 and 3 phase clinical trials also proved its efficiency and safety in a multitude of hematologic and inflammatory diseases including ulcerative colitis, rheumatoid arthritis and AD [12–14]. Miao aimed to evaluate the efficacy of JAK inhibitors for the treatment of AD by meta-analysis. According to its subgroup-analysis, upadacitinib for 4 weeks was effective treatment based on the EASI score. However, a small sample size and paucity of sufficient trials included may decrease its reliability [15]. So more evidence is needed to draw robust conclusions.
Aim
This meta-analysis aimed to assess available trials on the efficacy and safety of upadacitinib in the treatment of moderate-to-severe AD, which may provide more robust evidence to facilitate clinical decision-making.
Material and methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. It is also registered in PROSPERO (http://www.crd.york.ac.uk/prospero) with registration number CRD 42023407550.
Search strategy and study selection
Our search strategy aimed to find all published literature up to 22 October 2022 related to AD and upadacitinib, so we combined MeSH words with free words to identify the related literature. We used the following algorithm (“Dermatitis, Atopic”[MeSH] OR Atopic Dermatitides OR Atopic Dermatitis OR Dermatitides, Atopic OR Neurodermatitis, Atopic OR Atopic Neurodermatitides OR Atopic Neurodermatitis OR Neurodermatitides, Atopic OR Neurodermatitis, Disseminated OR Disseminated Neurodermatitides OR Disseminated Neurodermatitis OR Neurodermatitides, Disseminated OR Eczema, Atopic OR Atopic Eczema OR Eczema, Infantile OR Infantile Eczema) AND (“upadacitinib”[Supplementary Concept] OR “Upadacitinib”[Title/Abstract] OR “ABT494”[Title/Abstract] OR “Rinvoq”[Title/Abstract]). We searched the following databases: PubMed, Embase, Web of Science, and Cochrane. Two of us (Y.H. and L.C.) independently screened studies for inclusion. Any discrepancy was resolved by discussion and consensus.
Inclusion and exclusion criteria
We comprised all trials on upadacitinib administration to treat AD based on the following inclusion criteria: 1. Eligible patients were adolescents (aged 12–17 years) or adults (aged 18–75 years) with diagnosed moderate-to-severe AD. Moderate-to-severe AD was defined as an Eczema Area and Severity Index (EASI) score of ≥ 16, a validated Investigator Global Assessment for Atopic Dermatitis (IGA-AD) score of ≥ 3, an AD involvement of ≥ 10% of body surface area, and a weekly average of daily Worst Pruritus Numerical Rating Scale (NRS) score of ≥ 4. 2. RCTS. 3. Reporting the following outcomes: EASI scores, IGA, the pruritus numeric rating scale (NRS), EASI-75, adverse event (AEs), and serious adverse events (SAEs). We excluded duplicate publications, conference abstracts, case reports, case series, letters to editors, reviews, and unrelated studies.
Data extraction
We extracted the following information: reference of study, study design, country, intervention details, outcome measures, and Clinical Trial Identifier. Efficacy outcomes included: improvement in EASI from baseline, the proportion of patients achieving IGA response from baseline (IGA = 0 [clear] or IGA = 1 [almost clear]), change from baseline in peak NRS scores, the proportion of patients who had achieved at least a 75% improvement in EASI score from baseline (EASI-75). Safety outcomes included AEs and SAEs.
Assessment of risk of bias
Two of us (X.W. and C.C.) independently assessed the risk of bias in each included study using the Cochrane Collaboration’s risk-of-bias assessment tool [17]. Disagreements were resolved by discussion and consensus.
Statistical analysis
The statistical analysis was carried out by Review Manager, version 5.4 software (The Cochrane Collaboration). We calculated standardized mean differences (SMD) with 95% confidence interval (CI) for continuous data and relative risk (RR) with 95% CI for dichotomous outcomes. Heterogeneity was assessed using I2 statistic. The fixed-effects model was used if no heterogeneity was present. Otherwise, the data were assessed by the random-effects model (I2 > 50%) [18]. The subgroup analysis was conducted based on different treatment dosages. The funnel plot was used to assess publication bias.
Results
Search results and characteristics of included studies
We initially identified 472 studies in 4 electronic databases and excluded those that did not meet the inclusion criteria, and five clinical trials published in four articles were identified for further evaluation [14, 19–21]. The flowchart for screening studies is shown in Figure 1.
In the five trials, a total of 3975 patients were enrolled. Two trials [20, 21] used combination therapy with topical corticosteroids, while others received upadacitinib monotherapy. Among the five trials, one was conducted in Japan [21], and the rest were multi-nation, including Europe, North and South America, Oceania, and the Asia-Pacific region. Detailed characteristics of included studies are presented in Table 1.
Table 1
Characteristics of the 5 included trials
Efficacy
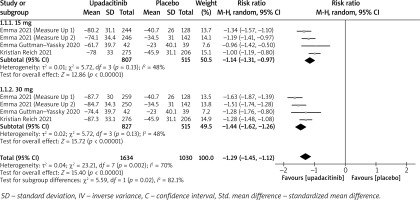
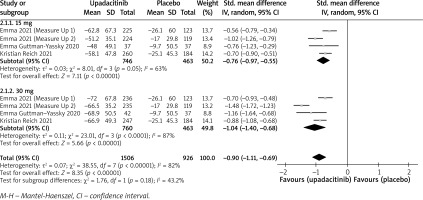
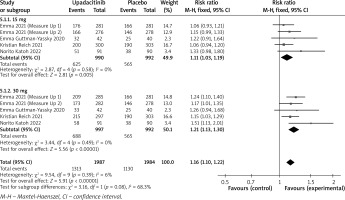
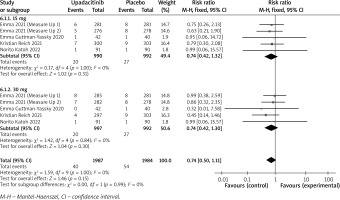
In terms of efficacy, we analyzed efficiency measures such as EASI scores, IGA, EASI-75, and pruritus NRS scores. Detailed endpoints used in this meta-analysis are presented in Table 2. Overall, our meta-analysis demonstrated significant improvement in the efficacy of upadacitinib for the treatment of AD in all measures of clinical indexes. The pooled analysis showed that upadacitinib treatment was associated with a significant reduction in EASI scores (SMD = –1.29; 95% CI: –1.45 to –1.12; p < 0.001), and significant heterogeneity was observed (I2 = 70%; p = 0.002) (Figure 2). Subgroup analysis related to different doses showed that 30 mg of upadacitinib seemed more effective than 15 mg of upadacitinib in the reduction of EASI (30 mg: SMD = –1.44; 95% CI: –1.62 to –1.26; 15 mg: SMD = –1.14; 95% CI: –1.31 to –0.97). The outcome of an investigator’s global assessment (IGA) of clear or almost clear skin revealed that patients treated with upadacitinib achieved significantly higher response rates compared to the placebo group (RR = 6.43; 95% CI: 5.01 to 8.26; p = 0.03) (Figure 3). Between the two dosages, 30 mg of upadacitinib demonstrated a higher response than 15 mg of upadacitinib in IGA (30 mg: RR = 7.35; 95% CI: 5.36 to 10.09; 15 mg: RR = 5.54; 95% CI: 3.86 to 7.96). Four studies reported Itch NRS improvement with a total of 2152 patients. The heterogeneity (I2 = 82%; p < 0.001) is statistically significant, so we used the random-effects model to analyse the data. Compared to placebo treatment, our analysis demonstrated that upadacitinib treatment significantly improved NRS scores (SMD = –0.9; 95% CI: –1.11 to –0.69; p < 0.001) (Figure 4). In the subgroup analysis, the 30 mg arm and 15 mg arm showed a significant reduction in Itch NRS compared to the placebo arm (30 mg: SMD = –1.04; 95% CI: –1.40 to –0.68; 15 mg: SMD = –0.76; 95% CI: –0.97 to –0.55). As for EASI-75, a higher proportion of patients achieving EASI-75 was found in upadacitinib treatment groups (RR = 3.97; 95% CI: 3.21 to 4.52; p < 0.001) (Figure 5). Nevertheless, the heterogeneity was statistically significant (I2 = 75%; p < 0.001). In the subgroup analysis, 30 mg of upadacitinib achieved a higher response than 15 mg in EASI-75 (30 mg: RR = 4.34; 95% CI: 3.16 to 5.98; 15 mg: RR = 3.65; 95% CI: 2.66 to 5.02).
Figure 3
Forest plot of the effect of upadacitinib treatment on proportion of patients achieving IGA response vs. placebo. Random-effects model
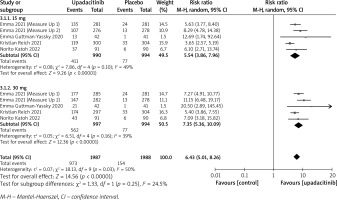
Figure 5
Forest plot of the effect of upadacitinib treatment on proportion of patients achieving EASI-75 vs. placebo. Random-effects model
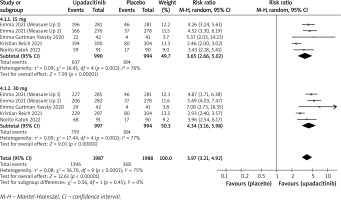
Table 2
Detailed endpoints related to this meta-analysis
Safety
All trials reported AEs and SAEs. Detailed AEs of the 5 included trials are presented in Table 3. The pooled analysis showed that the incidence of AEs was different between the treatment group and placebo group (RR = 1.16; 95% CI: 1.10 to 1.22) (Figure 6) with a minimal heterogeneity 3 (I2 = 6%; p = 0.39). In the 30 mg and 15 mg groups, the rates of patients with AEs were 50.1% (RR = 1.21; 95% CI: 1.13 to 1.30) and 49.9% (RR = 1.11; 95% CI: 1.03 to 1.19), respectively. The most common AEs reported included acne, upper respiratory tract infection, nasopharyngitis, and headache [19]. These AEs were reported to be mild to moderate.
Table 3
Detailed AEs of the 5 included trials
Looking at SAEs, no significant differences were found between the treatment group and placebo group (RR = 0.74; 95% CI: 0.50 to 1.11) (Figure 7). There was no statistically significant heterogeneity between studies (I2 = 0%; p = 1.00).
Risk of bias in included studies
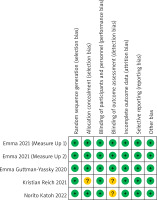
Figure 8 shows the risk of bias within all included RCTs for all outcomes, as judged by two researchers. The studies included were considered to have a low and unclear risk of bias.
All of the 5 trials used random sequence generation, and these trials were described as being double-blinded and detailed data were available. While blinding of outcome assessment was not mentioned in 2 trials [20, 21]. Because the included studies were less than ten, we did not perform the test of publication bias.
Discussion
The meta-analysis investigated the efficacy and safety of two dosages of upadacitinib in the treatment of patients with moderate-to-severe AD across 5 trials. The results demonstrated that both doses of upadacitinib were efficient in the treatment of moderate-to-severe AD with acceptable and mild AEs.
According to the data we collected, we chose the EASI score, EASI-75, IGA and NRS score to evaluate the treatment efficiency of upadacitinib. EASI and IGA response focus more on the basic characteristics of localized lesions, assessing the extent of the disease by considering eczema, induration, excoriation, and lichenification. In contrast, NRS is used to evaluate itch intensity in patients with moderate-to-severe AD. Across all the included studies, upadacitinib significantly improved local and general physical signs in patients with moderate to severe AD. According to the results of the subgroup analysis, 30 mg of upadacitinib was more efficient in the objective and subjective symptom improvement compared to 15 mg of upadacitinib.
The upadacitinib treatment displayed a favourable safety profile. According to our meta-analysis, patients treated with upadacitinib experienced a higher incidence of AEs compared to the placebo group. The most frequently reported AEs were acne, upper respiratory tract infection, nasopharyngitis, headache, and elevation in plasma creatine phosphokinase levels. These AEs were usually mild and tolerated. Similar to other JAK inhibitors for AD, such as abrocitinib and baricitinib [22, 23], the most frequently reported adverse event in clinical trials is acne. The acne lesions mainly consisted of inflammatory papules, pustules, and comedones, with only a few cases of cysts and nodules [19]. All events were non-serious. When compared to the placebo group, a higher incidence of eczema herpeticum was observed in both the upadacitinib 15 mg and 30 mg treatment groups. Regarding hepatic disorders, most cases reported were asymptomatic elevations in aminotransferase levels, which were temporary and did not require discontinuation of the study medication. However, special attention should be given to the occurrence of neutropenia. The incidence of neutropenia was higher in the treatment group compared to the placebo group, and in 1 case, a patient in the upadacitinib 30 mg plus topical corticosteroids group had to discontinue treatment due to this side effect [20].
As for the serious adverse event rates, there was no significant difference between the treatment group and the placebo group. In Measure Up 1 and Measure Up 2 [19], 6 cases of malignancy were reported in the upadacitinib groups. However, it is important to note that all of these malignancies were diagnosed within 65 days, suggesting that they were not related to the use of upadacitinib.
This meta-analysis also has some limitations. First, the number of patients included is small. Second, as a chronic inflammatory disease, it is necessary to evaluate treatment efficiency at different time points. Long-term studies are also needed to assess its safety, including the risk of cardiovascular disease and melanoma. Third, significant heterogeneity existed among trials. The possible sources of heterogeneity might contribute to different geographic regions or races. Furthermore, the measurement tools used are also a limitation. The Scoring Atopic Dermatitis and the Eczema Area and Severity Index (SCOREAD), which are considered to be validated measurement tools for the assessment of the severity of AD, were not used for no sufficient data. Finally, we also need more studies to compare different JAK inhibitors with each other. It is worth noting that Wan et al. compare the efficacy and safety of abrocitinib, baricitinib, and upadacitinib for moderate-to-severe AD by conducting a network meta-analysis [24]. They demonstrated that upadacitinib 30 mg was superior to all regimens in terms of IGA and EASI response. However, a simple assessment of lesions does not represent a comprehensive evaluation of AD severity, we also need to further evaluate patient-assessable instruments such as NRS and QOL.
Conclusions
This meta-analysis demonstrated that upadacitinib was a promising therapeutic option for AD due to its proven efficacy in alleviating the signs and symptoms. Adverse events are mild and acceptable. In the subgroup analysis, 30 mg doses of upadacitinib appear to be more effective for moderate-to-severe AD patients than 15 mg doses of upadacitinib. More trials are needed to further identify the long-term efficacy and safety of both two doses of dupilumab in moderate-to-severe AD for providing detailed evidence about the drug.