Introduction
Crohn’s disease (CD) is a type of inflammatory bowel disease (IBD) characterized by granulomatous, chronically recurrent gastrointestinal inflammation that tends to occur in the terminal ileum and colon [1, 2]. Over time, nearly half of patients develop complications (strictures, perianal disease, fistula or abscess), often resulting in surgical interventions [3, 4].
Crohn’s disease occurs more frequently in developed countries in North America and Western Europe [5]. Since the 21st century, the incidence of CD has gradually increased globally, especially in some emerging industrialized countries in Asia, South America, and Africa [6]. Currently, the goal of treating CD is to induce remission in the short term and maintain it in the long term. Pharmacological treatments include corticosteroids, thiopurine, methotrexate, and biological therapies. The introduction of biologics has brought considerable efficacy to the management of CD, along with a better safety profile than corticosteroids and immunosuppressants/immunosuppressive agents [2, 7]. However, some inhibitors, such as tumor necrosis factor (TNF) inhibitors, still have drawbacks in the treatment of CD, primarily non-response, loss of secondary reaction, potential serious adverse events (SAEs), and treatment-related costs [8-10].
The IL--23 pathway has been implicated in the pathogenesis of several immune-mediated chronic inflammatory diseases including psoriasis and CD [11, 12]. Also, biologics such as IL-23 inhibitors and IL-12/23 inhibitors have been shown to be effective in the treatment of patients with CD in randomized controlled trials (RCTs), both in the induction period and maintenance period. With the continuous emergence of the IL-23 inhibitors, there has not been a comprehensive analysis of the research in this area. In the present study, the efficacy of IL-23 and IL-12/23 inhibitors in the induction phase for the treatment of CD was further evaluated through a meta-analysis based on published randomized controlled trials.
Material and methods
Search strategy
This study was carried out according to the latest Preferred Reporting Items for Systematic review and Meta- Analysis (PRISMA) 2020 guidelines [13]. A comprehensive systematic search of the literature was conducted from inception until December, 2022, using Medline, Embase, Web of Science and the Cochrane Library. Any potential RCTs relevant to the efficacy of IL-23 inhibitors or IL-12/23 inhibitors in the treatment of CD would be adopted. The search algorithms were as the follows: ((Interleukin-23) OR (Interleukin 23) OR (IL-23) OR (IL 23) OR (Interleukin-12) OR (Interleukin 12) OR (IL-12) OR (IL 12)) AND ((Crohn’s Enteritis) OR (Crohn Disease) OR (Regional Enteritis) OR (Crohn’s Disease) OR (Crohns Disease) OR (Inflammatory Bowel Disease 1) OR (Enteritis, Granulomatous) OR (Granulomatous Enteritis) OR (Colitis, Granulomatous) OR (Granulomatous Colitis)). The literature search was conducted independently by two experienced researchers in systematic reviews.
Study selection
The study selection was based on the PICOS (participants, intervention, comparison, outcomes and study design) principle. Studies that met the following criteria were considered eligible: 1) participants: patients aged 18 years or older who were suffering from CD; 2) intervention: IL-23 inhibitors or IL-12/23 inhibitors (alone or combined with other therapies) in the induction period; 3) comparison: placebo and/or other non-biological treatments; 4) outcomes: improvement/normalization of symptoms (Crohn’s Disease Activity Index – CDAI) and/or biochemical markers and/or endoscopic findings; 5) study design: phase II/III RCTs.
The exclusion criteria were as follows: 1) studies limited to maintenance therapy; 2) the data were unavailable or missing; 3) articles not published in English; 4) studies including pediatric patients. For updated duplicated articles, the latest published or the studies with largest sample sizes were included in the study.
Data extraction
Two investigators independently completed the extraction according to the pre-specified formats, and discrepancies were ironed out through discussion. The following study characteristics were extracted: author, year of publication, country, trial phase, participants’ characteristics (e.g., age, gender, disease duration, biological therapy experience), number of patients, treatment regimen, follow-up duration and reported outcomes.
The primary outcome of the induction therapy was achievement of clinical remission (defined by a CDAI < 150). Secondary endpoints included achievement of clinical response (defined by a reduction in the CDAI ≥ 100 points compared to baseline), endoscopic remission, endoscopic response and normalization of C-reactive protein (CRP).
Quality assessment
The Cochrane Collaboration’s tool [14] was used to assess the risk of bias inherent in the included RCTs. The inherent risk of bias included random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other biases. Each aspect was scored as “low risk”, “high risk”, or “unclear risk”, with low overall risk indicating that the study was less prone to bias.
Statistical analysis
RevMan version 5.4 software was used in the statistical analysis. For dichotomous variables, the effect size was calculated using relative risk (RR) and the corresponding 95% confidence interval (CI). The heterogeneity between studies was evaluated using the I2 statistic: a value of 0% indicates no observed heterogeneity, and a larger value denotes increased heterogeneity [15]. Given the complexity of patient baseline characteristics and the use of different biological inhibitors, a random-effects model was used to analyze the results of the included studies. In order to increase the credibility of the results, publication bias (Egger’s weighted regression test [16]) and sensitivity analyses were performed since more than 10 studies were included. Moreover, a two-sided p-value < 0.05 for all statistical outcomes described above was considered statistically significant.
Results
Study selection
Based on our literature search, a total of 944 studies were identified. After excluding duplicates, 524 studies remained, 15 of which were considered potentially relevant and were read in full text. Among the 15 studies, 1 was excluded because the study protocol did not meet the inclusion criteria, 4 were excluded because no outcomes of interest were reported and 3 were excluded because the dates were unavailable. Finally, 7 studies containing 3,990 patients were included in the meta-analysis. The flowchart for the search procedure is displayed in Figure 1.
Study characteristics and quality assessment
The characteristics of the selected RCTs are shown in Table 1. Of these 7 studies, 5 RCTs evaluated the induction of IL-23 inhibitors (including risankizumab, guselkumab, mirikizumab and brazikumab) [17-21], and 3 RCTs evaluated the induction of IL-12/23 inhibitors (ustekinumab only) [18, 22, 23]. Notably, one of the studies evaluated both IL-23 inhibitors and IL-12/23 inhibitors. All intervention groups were treated with a single IL-23 inhibitor or IL-12/23 inhibitor, while control groups were placebo-controlled only. Clinical remission and clinical response were reported in all studies. In addition, among the research of IL-23 induced therapy, 3 studies reported endoscopic remission rates, and 4 studies reported endoscopic response rates. The IL-23 inhibitors were assessed at week 12, while IL-12/23 inhibitors were assessed at week 8.
Table 1
Baseline characteristics of studies included in the meta-analysis
The mean age of patients ranged from 35 to 40 years. The mean duration of CD history in patients across studies ranged from 8.8 to 13 years. Most patients had been treated with TNF inhibitors. For more information, see Supplementary Table 1.
Supplementary Figs. 1 and 2 show the results of the quality assessment. In the meta-analysis, the 7 studies were appraised for quality using the Cochrane Collaboration’s tool and all were judged to be of low overall risk and high quality.
Analysis of IL-23 or IL-12/23 inhibitors for the CD patients
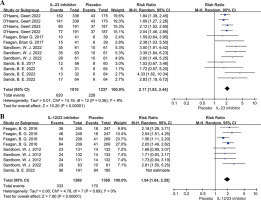
For the primary outcome, patients receiving IL-23 inhibitors showed a higher clinical remission rate than patients with placebo therapy (RR = 2.11, 95% CI: 1.83-2.44, p < 0.001, I2 = 9%; Fig. 2A), and patients receiving IL-12/23 inhibitors also had a higher clinical remission rate than the placebo group (RR = 1.94, 95% CI: 1.64-2.29, p < 0.001; Fig. 2B). In terms of the second endpoints, both the IL-23 inhibitor group and the IL-12/23 inhibitor group showed significantly higher clinical response rates compared to the placebo group (RR = 1.92, 95% CI: 1.74-2.11; RR = 1.83, 95% CI: 1.61-2.09; respectively, Fig. 3). In addition, the IL-23 inhibitor group had a higher endoscopic remission rate and endoscopic response rate than placebo group, with a corresponding pooled RR of 3.40 (95% CI: 2.57-4.50, p < 0.001; Fig. 4) and 2.65 (95% CI: 2.65-3.12, p < 0.001; Fig. 5), respectively. Moreover, a higher proportion of patients in the IL-23 inhibitor group and IL-12/23 inhibitor group achieved CRP normalization compared to the placebo group (RR = 5.76, 95% CI: 4.10-8.11; RR = 2.38, 95% CI: 1.86-3.04; respectively, Fig. 6).
Discussion
In this study, we analyzed the efficacy of IL-23 inhibitors and IL-12/23 inhibitors in the induction phase for the treatment of CD. To our knowledge, this is the first systematic review and meta-analysis evaluating the effectiveness of IL-23 inhibitors in the induction phase of CD treatment. Although there have been previous meta-analyses on IL-12/23 inhibitors [24], this review was updated based on high-quality RCTs. Overall, our analysis validates the good performance of IL-23 inhibitors and IL-12/23 inhibitors in the induction phase of treating CD in patients with or without TNF experience. Compared with placebo, more patients in the IL-23 and IL-12/23 inhibitor groups achieved clinical remission, clinical response and CRP normalization. Additionally, more patients treated with IL-23 inhibitors achieved endoscopic remission and an endoscopic response compared to those who received placebo intervention.
Interleukin 23 plays an important role in a variety of inflammation-mediated autoimmune diseases such as psoriasis, psoriatic arthritis, ankylosing spondylitis, systemic lupus erythematosus, and IBD [25-29]. Biologics targeting IL-23p19 and IL-12/23p40 subunits have been confirmed to be effective in the treatment of psoriasis and psoriatic arthritis [30-32]. IL-23 is a member of the IL-12 cytokine family and consists of the IL-23p19 subunit and the IL-12/23p40 subunit, which is shared with IL-12 [33]. Ustekinumab specifically blocks the IL-12/23 subunit p40 in CD patients whereas risankizumab, brazikumab, guselkumab and mirikizumab selectively block the unique subunit p19. Ultimately, they all lead to the blockade of the IL-23 signaling pathway and suppress the inflammatory response [12, 34].
In previous studies, results from animal experiments and genetic studies have highlighted the role of the IL-23/IL-17 axis in the pathogenesis of IBD. A significant and selective increase of IL-23-responsive innate lymphoid cells (ILCs) was found in the inflamed intestine in CD patients but not in ulcerative colitis (UC) patients, where ILCs may promote intestinal inflammation through cytokine production and lymphocyte recruitment [35-37]. In addition, there are T-helper-cell-17 cells, γδT cell subsets, and natural killer T cells that respond to IL-23, and an increase in these cells also promotes the intestinal inflammatory response in CD patients [38-40]. In conclusion, various lines of evidence have suggested that IL-23 is an effector cytokine in the innate gut immune system, which may be a more specific target for the treatment of CD. Additionally, preclinical studies have shown no benefit of selective blocking of IL-12 in IBD [41, 42], and other studies in animal models have demonstrated that blocking IL-23 is more important than IL-12 in suppressing intestinal inflammation [43-45]. Research by Chapuy et al. found that IL-12 and mucosal CD14 monocyte-like cells induce IL-8 in colonic memory CD4 T cells in UC patients, but not in CD patients. This may be relevant to the pathogenesis of UC and CD [46].
The natural history of CD is characterized by periods of remission and relapse, and the probability of gastrointestinal complications increases when intestinal inflammation has not been well controlled [3]. The risk of developing stenosis or penetrating disease within 5 years of diagnosis of CD was 33.7%. The cumulative incidence of perianal or rectovaginal fistula within 10 years of diagnosis is 18% [47, 48]. Two RCTs reported improved fistula closure rates with TNF inhibitor treatment compared to placebo. However, due to the high recurrence rate and side effects, the long-term effect of TNF inhibitors was less favorable [49-51]. It is therefore necessary to find better treatments for CD and its complications.
In this study, the efficacy of IL-12/23 and IL-23 inhibitors in the induction phase of CD was confirmed in terms of clinical manifestations, endoscopic response and inflammatory markers. Clinical symptoms of CD correlate poorly with the degree of mucosal inflammation, and it is common to find significant mucosal inflammation during complete clinical remission [52]. Endoscopic healing is considered to be a better indicator of the severity of inflammation of the intestinal mucosa. Therefore, researchers consider symptom relief (clinical response, clinical remission) to be a short-term and intermediate treatment goal, while treatment aimed at endoscopic healing is more reflective of long-term outcomes and may reduce the risk of bowel injury [53, 54]. Fecal calprotectin and CRP were the two most widely used biomarkers in IBD, fecal calprotectin has high sensitivity and low specificity in identifying mucosal inflammation, whereas CRP has the opposite profile. Therefore, a normal CRP after initiating treatment should be considered as a minimal obligatory short- to medium-term target [53].
Treatment of CD includes induction therapy during an acute phase of the attack and long-term maintenance therapy. Therapeutic agents during the induction phase include higher doses of corticosteroids and biological therapies. However, with corticosteroids, adverse effects (especially metabolic bone diseases, infectious complications and growth disorders [55]) and complications associated with long-term use limit their use during the maintenance phase. In the maintenance phase, treatment options include immunomodulators (azathioprine, 6-mercaptopurine, and methotrexate) and biological therapies (TNF-α, IL-12/23, IL-23 and integrin α4β7).
At present, the “step-up” strategy (the introduction of biological therapies when multiple traditional therapeutic agents have failed) is still widely used in clinical practice [7, 56]. However, recent studies have shown that early biological therapy (mainly anti-TNF therapy) may improve the prognosis of patients and prevent progression to irreversible bowel damage [2, 57-59]. Berg et al. stated that the benefits of early biologics for CD were beneficial to control inflammation and avoid progression to irreversible stricture and penetrating disease. In contrast, patients with UC have predominantly mucosal rather than transmural inflammation and complications such as strictures are rare [7]. According to the British Society of Gastroenterology consensus guidelines, patients with jejunum or extensive small bowel disease have a poor prognosis, and early use of biological therapies may be considered. It also recommended that ustekinumab could be used in the induction and maintenance period of CD, both in anti-TNF naive patients and in patients who have failed anti-TNF treatment [60, 61]. This is despite growing evidence that IL-23 inhibitors are effective and safe for the treatment of CD. However, the use of IL-23 inhibitors was not explicitly recommended in recently published guidelines [2, 57, 60]. For the treatment of advanced severe CD, a head-to-head RCT showed that laparoscopic ileocecal resection is more medically effective and more cost-effective in treating end-stage ileitis in CD compared with biological therapies [62, 63].
There are some limitations to the present study. Firstly, IL-12/23 inhibitors other than ustekinumab were not included as data could not be extracted. Secondly, because the study focused on evaluating the efficacy of IL-23 and IL-12/23 inhibitors in the induction phase for the treatment of CD, the efficacy of long-term maintenance therapy is unknown. Finally, due to differences in study design among the included RCTs, the efficacy of IL-23 and IL-12/23 inhibitors in the induction period was not directly compared.
Conclusions
In this systematic review and meta-analysis, IL-12/23 inhibitors and IL-23 inhibitors were shown to be effective in the induction phase in the treatment of patients with CD, as evidenced by three aspects: clinical symptoms, endoscopic symptoms, and inflammatory markers. The IL-23 and IL-12/23 inhibitors have the potential to be a promising therapeutic option for CD patients who have had insufficient responses to conventional and TNF therapies.