Introduction
Pulmonary arterial hypertension (PAH) is a complex and progressive condition characterized by the elevation of blood pressure within the pulmonary arteries, leading to significant morbidity and mortality [1]. This pathological state poses a substantial challenge in clinical management due to its multifactorial nature and the wide spectrum of underlying causes [2]. The development of effective therapeutic strategies is crucial in mitigating the progression of the disease and improving patient outcomes [3]. Among the various pharmacological interventions available, treprostinil, a synthetic analogue of prostacyclin, has emerged as a promising agent in the treatment landscape of pulmonary hypertension [4].
In this systematic review and meta-analysis, we aim to comprehensively analyze the available literature to evaluate the clinical effectiveness of treprostinil in patients with pulmonary hypertension. Our focus is on assessing the impact of treprostinil on key clinical outcomes, including exercise capacity, adverse events, functional class, and overall survival.
Methods
Study design
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We designed the study to evaluate the efficacy and safety of treprostinil in the treatment of pulmonary hypertension.
Search strategy
A comprehensive literature search was performed across several electronic databases, including PubMed, Embase, Web of Science, Scopus, and the Cochrane Library. The search was conducted without language restrictions and covered all available literature up to January 2024. Keywords and MeSH terms related to “pulmonary hypertension”, “treprostinil”, and “clinical trials” were used. Reference lists of identified articles were also manually searched for additional relevant studies.
Inclusion and exclusion criteria
Studies were included if they were randomized controlled trials (RCTs), cohort studies, or case-control studies evaluating the efficacy and/or safety of treprostinil in patients with pulmonary hypertension. Studies were excluded if they were non-comparative, had a sample size of less than 10 patients, or if full-text articles were not accessible. Reviews, editorials, and animal studies were also excluded.
Data extraction
Two independent reviewers extracted data from the included studies. Discrepancies were resolved through discussion or consultation with a third reviewer. Extracted data included study characteristics (author, year of publication, study design), participant demographics, details of treprostinil treatment (dose, route of administration), comparator details (if any), and outcomes (including hemodynamic parameters, exercise capacity, functional class, and adverse events).
Quality assessment
The quality of the included studies was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials. Each study was evaluated for selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential sources of bias.
Statistical analysis
Meta-analysis was performed using the software Comprehensive Meta-Analysis (CMA). The effect size for continuous outcomes was expressed as mean differences or standardized mean differences with 95% confidence intervals (CIs), while for dichotomous data, risk ratios or odds ratios with 95% CIs were used. Heterogeneity among studies was assessed using the I² statistic. A random-effects model was applied in cases of significant heterogeneity (I² > 50%), while a fixed-effects model was used otherwise. Sensitivity analysis was conducted to explore the influence of individual studies on the overall results. Publication bias was assessed using funnel plots and Egger’s regression test.
Results
Study selection
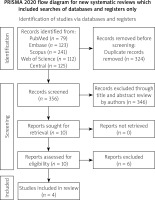
Our comprehensive search yielded a total of 680 records. After the removal of 324 duplicates, 356 records were screened for eligibility, leading to the retrieval of 10 full-text articles for detailed assessment. Ultimately, four studies were included in our systematic review and meta-analysis [5–8]. The PRISMA flow diagram (Figure 1) illustrates the study selection process, highlighting the rigorous screening and inclusion criteria applied.
Study characteristics
Three studies compared combined treprostinil therapy with the patients’ previous treatment against a combined placebo with their previous treatment [5, 7, 8]. The remaining study directly compared treprostinil against a placebo alone [6]. The characteristics of these studies, including patient demographics, baseline disease status, previous treatments, and treprostinil dosing regimens, are summarized in Table I.
Table I
Characteristics of included studies
The included studies were conducted across a wide range of centers. The patient cohorts were large, with sample sizes ranging from 310 to 690. The mean age of participants varied from 41.2 years to 50.9 years, with a predominance of female patients, accounting for 75% to 82% of the study populations. This demographic spread is reflective of the known epidemiology of pulmonary hypertension, which tends to be more prevalent in middle-aged females. A significant proportion of patients in these studies had been diagnosed with idiopathic pulmonary arterial hypertension (IPAH), ranging from 63% to 74%. Furthermore, the studies included patients with advanced disease, as evidenced by a high percentage of individuals with a functional class (FC) greater than III. Regarding treatment history, participants had diverse backgrounds of previous therapies, including phosphodiesterase type 5 (PDE5) inhibitors, endothelin receptor antagonists (ERA), or both. The duration from diagnosis to the initiation of treprostinil treatment varied across the studies, from 1 to 3.9 years, with follow-up periods of 12 to 60 months. The mean treprostinil dosages administered in the studies ranged from 0.63 mg twice daily to 3.5 mg three times daily.
Risk of bias
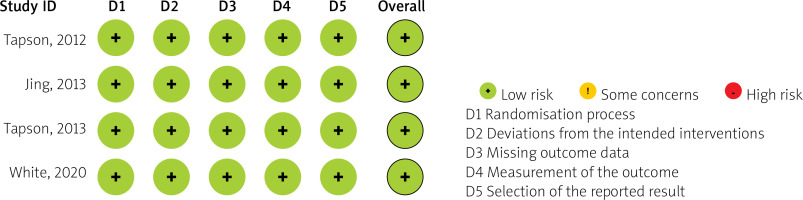
The risk of bias among the included studies was comprehensively assessed and is detailed in Figure 2. Overall, the studies demonstrated a low risk of bias across key domains, including selection, performance, detection, attrition, and reporting biases.
Outcomes
Exercise capacity
The primary efficacy outcome, exercise capacity, was measured by the 6-minute walk distance (6MWD). The meta-analysis revealed a mean difference of 13.13 m in favor of treprostinil over the control, indicating a significant improvement in exercise capacity with treprostinil treatment (I² = 99%; Figure 3 A). This high degree of heterogeneity suggests variability in the effect size across studies. However, during sensitivity analysis, where treprostinil monotherapy was excluded, the mean difference in 6MWD remained significant at 8.13 m in favor of treprostinil, with heterogeneity dropping to 0% (Figure 3 B).
Figure 3
A – Forest plot of the effect of treprostinil on exercise capacity (6MWD), B – sensitivity analysis forest plot for exercise capacity (6MWD) excluding treprostinil monotherapy, C – forest plot of adverse events leading to discontinuation, D – forest plot of the risk of clinical worsening, E – mortality rates in treprostinil vs. control groups

Safety and tolerability
In terms of safety, the incidence of adverse events leading to discontinuation was higher in the treprostinil group, with an odds ratio (OR) of 4.39 (I² = 0%; Figure 3 C).
Clinical worsening
Analysis of the risk of clinical worsening demonstrated a protective effect of treprostinil, with an OR of 0.554 in favor of treprostinil (I² = 0%; Figure 3 D).
Mortality
The occurrence of death did not differ significantly between the treprostinil and control groups, with an I² of 0%, indicating no effect of treprostinil on mortality (p = 0.93; Figure 3 E).
Discussion
This systematic review and meta-analysis rigorously evaluated the efficacy and safety of oral treprostinil in treating PAH, a condition that significantly challenges patient management and outcomes. Our findings suggest that treprostinil offers a promising therapeutic benefit, particularly in improving exercise capacity as measured by the 6MWD. The mean difference of 13.13 m in favor of treprostinil, despite the high heterogeneity among studies, underscores a consistent benefit in exercise capacity. This improvement is critical for PAH patients, for whom diminished exercise tolerance is a marker of disease progression and a determinant of quality of life [9–11].
In the context of evaluating treatments for PAH, the minimal clinically important difference (MCID) for the 6MWD is recognized to be approximately 30 m [12]. Our analysis, however, revealed a mean improvement of 13 m in 6MWD among patients treated with oral treprostinil compared to control groups. Although this improvement falls short of the MCID, it nonetheless indicates enhanced exercise capacity in this patient population. The discrepancy between the observed improvement and the MCID highlights the need for cautious interpretation of the results, suggesting that while oral treprostinil may beneficially affect exercise capacity, the effect may not reach the threshold considered clinically significant by current standards.
Our results are in line with the pharmacological profile of treprostinil, which acts as a vasodilator and inhibits platelet aggregation, thereby potentially improving pulmonary arterial pressure and resistance, which in turn could enhance physical endurance [13].
Safety and tolerability are paramount in evaluating new or existing treatments, given the chronic nature of the disease and the need for long-term therapy [14]. Our analysis revealed an increased incidence of adverse events leading to discontinuation in the treprostinil group. This finding is consistent with the known side effect profile of prostacyclin analogues, which includes headache, diarrhea, nausea, and jaw pain [15–17]. The higher OR of 4.39 for discontinuation due to adverse events highlights the necessity for careful patient selection, dose titration, and management of side effects in clinical practice [18, 19].
The protective effect of treprostinil against clinical worsening, with an OR of 0.554, is particularly noteworthy. This outcome suggests that beyond improving exercise capacity, treprostinil may offer benefits in slowing the progression of PAH, a key goal in the management of this disease [20]. However, the absence of a significant impact on mortality observed in this analysis indicates the complexity of PAH management and the need for comprehensive treatment strategies that may include combination therapy.
Limitations: This review is not without limitations. The small number of included studies and the variability in their design and reporting limit the generalizability of the findings. The lack of significant publication bias strengthens the validity of our conclusions, suggesting that our findings are representative of the available evidence and not unduly influenced by unpublished negative studies.
Clinical implications: Our findings support the use of oral treprostinil as a valuable option in the therapeutic arsenal against PAH, particularly for improving exercise capacity. Clinicians should weigh the benefits of treprostinil against its side effect profile and consider it as part of a multidisciplinary approach to managing PAH. The potential of treprostinil to reduce clinical worsening further emphasizes its role in comprehensive patient care strategies.
Conclusions
Oral treprostinil represents a promising treatment for PAH, offering significant benefits in exercise capacity and potentially delaying clinical worsening. Despite its challenges, including a higher rate of adverse events leading to treatment discontinuation, treprostinil’s role in improving patient outcomes warrants its consideration in the management of this complex disease. Further research is needed to fully elucidate its impact on long-term clinical outcomes, including quality of life and mortality, in the PAH patient population.