Introduction
Hepatic encephalopathy (HE) is a significant con-sequence of acute or chronic liver disease that is caused by metabolic abnormalities in the central nervous system. Moreover, minimal hepatic encephalopathy (MHE) is the mildest form of HE, indicated by the absence of obvious clinical impairment. It is characterized by mild motoric and cognitive impairment, and a decline in health-related quality of life (HRQL) [1].
The prevalence of MHE varies between 23.7% and 56.6% in patients with liver cirrhosis [2]. It is influenced by the previous history of overt HE (OHE), the severity of liver disease, age, alcoholic etiology, and the portosystemic shunt procedure. Moreover, MHE patients had a greater incidence of OHE and mortality than individuals without MHE [1]. Within three years, half of patients (50%) with MHE develop OHE, according to reports. Minimal HE can also interfere with daily activities, impair job performance and quality of life, and raise the chance of causing a car accident [2]. Early detection of MHE is critical for initiating timely treatment, enhancing cognitive function and quality of life, as well as preventing progression to OHE [1].
Treatment of MHE targets the gut due to the role of intestinal ammonia-producing bacteria that has been indicated to be involved in MHE pathogenesis [3]. Lactulose is considered a first-line treatment option for patients with HE, but it has some common side effects, e.g., diarrhea and flatulence, leading to poor patient compliance [4]. Rifaximin, a minimally absor-bed oral antibiotic, is effective primarily as a lactulose adjunct in some studies. Still, prolonged antibiotic use is correlated with a higher risk of infection with anti-biotic-resistant bacterial strains [5].
A small number of publications on probiotics have yielded favorable results regarding primary prevention of HE, risk of hospitalization due to HE, and severity of the liver disease [6, 7]. Probiotics are living microorganisms that affect the gut microbiota balance [3]. It is believed that the mechanism of action involves modulation of the gut microbiome and metabolism [5]. This modality is expected to be used as a long-term treatment in patients with HE [8].
To the authors’ best knowledge, there are three meta-analyses analyzing the efficacy of probiotics for MHE treatment [9-11], but their studies are limited to studies up to 2015, whereas other meta-analyses investigated the use of probiotics for HE but not specifically for MHE. Additional studies after 2015 with considerable results need to be evaluated. This review aimed to provide an additional evaluation regarding the efficacy of probiotics in MHE patients.
Material and methods
This meta-analysis conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards.
Literature search
The eligibility criteria were established through implementation of the PICO (patient, intervention, comparison, and outcome) concept. Table 1 depicts the PICO framework used for this study. Using the Boolean operator, the PICO eligibility criteria were extracted as keywords. In the present study, we employed the keywords “cirrhosis” AND “probiotics” AND (“mini-mal hepatic encephalopathy” OR “mild hepatic encephalopathy” OR “minimal HE”) in PubMed, Science Direct, and Cochrane Library to find eligible journals. Additionally, we examined the references of pertinent articles. Duplicate results were removed after the initial search. The last search was run on September 10th, 2022.
Table 1
PICO framework of the study
| Variable | |
|---|---|
| Patient | Cirrhotic patients with MHE |
| Intervention | Probiotics |
| Comparator | Other treatment (lactulose, rifaximin, LOLA, etc.) |
| Outcome | Improvement of MHE |
The publications were included if they matched all of the mentioned criteria: 1) randomized controlled trials (RCTs) reporting patients with MHE, 2) adult patients, 3) reporting intervention with probiotics compared with other comparators (lactulose, rifaximin, LOLA, etc.), 4) articles in English or Indonesian. Moreover, a study was excluded if it met any of the following criteria: 1) case report, 2) abstracts only, 3) conference papers, 4) review articles, 5) non-research letters, 6) commentaries, 7) did not provide the necessary data for conducting a meta-analysis, and 8) no comparator involved in the studies.
Study selection
Four authors (IDNW, IKM, CPS, DAS) independently performed study selection. A screening of study titles and abstracts was undertaken to exclude irrelevant literature. The inclusion and exclusion criteria for this review were applied to publications that passed the initial screening.
Data extraction
Data of the included studies extracted are the author’s name, design of the study, sample size, duration of probiotics administration, and type of probiotics.
The primary outcome studied in the present systematic review and meta-analysis is reversal of MHE after probiotics intervention. The secondary outcome was reduction of serum ammonia and improvement of neuropsychometric tests. All authors utilized an electronic data collection form to acquire the necessary information from each article.
Risk of bias
The Cochrane Risk of Bias Tool was employed to assess the methodological quality of the research [12]. Two authors (IDNW and CPS) independently conducted this process, which aimed to minimize the possibility of study selection bias. The assessment was based on random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias.
Statistical analysis
A meta-analysis was undertaken using Review Manager 5.4 as the software. Reversal of MHE was assessed using odds ratio (OR) estimation with a 95% confidence interval (CI). The outcomes were pooled using the Mantel-Haenszel random effects model. Changes in serum ammonia levels and neuropsychometric test scores were examined using mean differences (MD) with 95% CI, and the findings were compared using an inverse variance random-effects model. We used a calculator by Luo et al. and Wan et al. to calculate the mean if data were given as median with Q1 and Q3 or range [13, 14]. Heterogeneity was examined using the I2 statistic, which indicates what proportion of the variation in observed effects across studies is attributable to the variation in true effects, with values > 60% suggesting significant heterogeneity. Sensitivity analysis was conducted with the leave-one-out method (removing one study each time and repeating the analysis). All p values were two-tailed, and < 0.05 was established as the threshold for statistical significance.
Results
Study selection and characteristics
The keywords search yielded a total of 62 publications. After eliminating the duplicates, we retrieved 48 publications. By screening the titles and abstracts, we excluded 33 studies, leaving us with 15 potential studies. Then, the full texts of the potential studies were obtained and reviewed to see if they were eligible for inclusion in the meta-analysis. Publications that did not offer all the necessary data for this meta-analysis and that did not fulfill all the inclusion criteria were excluded. Thereby, in the present study, a total of 9 studies including 776 patients were included [15-23].
The characteristics of the included literature can be seen in Table 2. Seven studies compared probiotics with placebo or no treatment [15-17, 19-21, 23]; three studies compared probiotics and lactulose [18, 19, 22]; two studies compared probiotics with LOLA [17, 19]; and only one study compared probiotics with rifaximin [24]. Each study used different probiotics and doses (Table 3).
Table 2
Characteristics of RCTs included in the meta-analysis
| Study | Duration | Intervention | Sample size | Age (mean ±SD) | Criteria used for diagnosis of MHE |
|---|---|---|---|---|---|
| Xia et al. (2018) [15] | 3 months | Probiotics No treatment | 30 37 | 41.5 ±12.9 43.8 ±10.3 | Abnormal NCT-A and DST results |
| Lunia et al. (2014) [16] | 3 months | Probiotics No treatment | 86 74 | 48.5 ±10.5 49.4 ±11.5 | PHES was –5 or less |
| Sharma et al. (2014) [17] | 2 months | Probiotics LOLA Rifaximin Placebo | 32 31 31 30 | 33.87 ±13.2 42.0 ±11.4 43.9 ±12.5 38.0 ±11.8 | 2 of the 3 psychometric tests were abnormal and/or CFFT was < 39 Hz |
| Sharma et al. (2008) [18] | 1 month | Probiotics Lactulose Lactulose + probiotics | 35 35 35 | 43.5 ±12.1 39.5 ±13.0 43.7 ±10.0 | Abnormal psychometric study (NCT A and NCT B or FCT A and FCT B) and/or abnormal P300ERP |
| Mittal et al. (2011) [19] | 3 months | Probiotics Lactulose LOLA No treatment | 34 35 32 31 | 41.2 ±11.9 43.85 ±10.9 42.15 ±8.7 44.25 ±11.8 | 2 or more abnormal psychometric tests (NCT/FCT A and B, BDT, picture completion test) |
| Bajaj et al. (2014) [20] | 2 months | Probiotics Placebo | 14 16 | 58.4 ±3.8 58.5 ±4.5 | At least 2 of the 4 of NCT A, NCT B, DST or BDT abnormal (NCT A > 35 seconds, NCT B > 99 seconds, DST < 72 or BDT < 31 raw score) |
| Bajaj et al. (2008) [21] | 2 months | Probiotics Placebo | 17 8 | 52 ±8 54 ±4 | Performance of NCT A or DST or BDT of Wechsler’s Adult Intelligence Scale-III was impaired 2 standard deviations (SD) beyond that of a group of 100 age- and educational status-matched community controls |
| Mouli et al. (2014) [22] | 2 months | Probiotics Lactulose | 33 40 | 39.6 ±11.4 44.2 ±10.4 | Positive neuropsychometric tests NCT A and B or FCT A and B for illiterates and/or positive neurophysiological test (P-300 auditory event-related potentials [P-300 ERP]) |
| Malaguarnera et al. (2007) [23] | 3 months | Probiotics Placebo | 30 30 | 46 ±11 45 ±12 | Presence of at least 1 abnormal psychometric test |
Table 3
Types and doses of probiotics in each included study
| Studies | Types of probiotics used | Doses |
|---|---|---|
| Xia et al. (2018) [15] | Clostridium butyricum and Bifidobacterium infantis | Dose of 1500 mg, 3 times daily for 3 months containing more than 1.0 × 107 CFU/g viable C. butyricum and more than 1 × 106 CFU/g viable B. infantis per capsule |
| Lunia et al. (2014) [16] | VSL#3 (containing Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus) | 1 capsule, 3 times per day |
| Sharma et al. (2014) [17] | Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus plantarum, Lactobacillus casei, Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium breve, Saccharomyces boulardii, and Streptococcus thermophilus | 2 capsules per day |
| Sharma et al. (2008) [18] | Streptococcus faecalis, Clostridium butyricum, Bacillus mesentericus, Lactic acid bacillus | 1 capsule 3 times per day |
| Mittal et al. (2011) [19] | Composition of probiotics used not available | 110 billion colony forming units, 2 times per day |
| Bajaj et al. (2014) [20] | Lactobacillus GG | Not clear |
| Bajaj et al. (2008) [21] | Probiotic yogurt (containing Streptococcus thermophilus, Lactobacillus bulgaricus, Lactobacillus acidophilus, Bifidobacteria, and Lactobacillus casei) | 12 ounces per day |
| Mouli et al. (2014) [22] | VSL#3 (containing Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus) | 2 capsule, 2 times per day (450 billion CFU/day) |
| Malaguarnera et al. (2007) [23] | Bifidobacterium longum | Not clear |
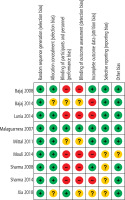
Risk of bias within studies
Two out of nine studies lacked adequate data to render a verdict on concealment of allocation. Five studies made no blinding of study personnel or participant and outcome assessors. Four studies have a high attrition rate (Fig. 1).
Probiotics compared with placebo or no treatmentEffect on reversal of MHE
Four studies compared the effect of probiotics on reversal of MHE with placebo or no treatment. Compared to placebo or no treatment, probiotics significantly reversed MHE with a pooled OR of 3.95 (p < 0.0001, 95% CI: 2.05 to 7.60) (Fig. 2A).
Fig. 2
Comparison of outcomes between probiotics group and placebo or no treatment group. A) Effect on reversal of MHE; B) Effect on improvement of CFFT; C) Effect on reduction of serum ammonia
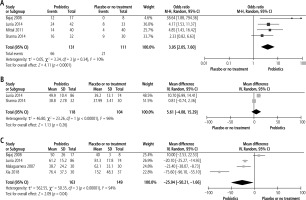
Effect on improvement of CFFT
Two studies compared CFFT between the probiotics group and placebo or no treatment group. There were no significant differences in CFFT improvement between the probiotics and placebo or no treatment group with a pooled mean difference of 5.61 (p = 0.26, 95% CI: –4.08 to 15.29) (Fig. 2B).
Effect on reduction of serum ammonia levels
There was a significant difference in the alleviation of serum ammonia levels at the end of studies between the probiotics and placebo or no treatment groups. Based on a random effect model (I2 = 94%, χ2 = 50.35, p < 0.00001), the pooled mean difference of serum ammonia levels (in µmol/l) at the end of the studies between the probiotics group and placebo or no treatment was –25.94 (p = 0.04, 95% CI: –50.21 to –1.66) (Fig. 2C).
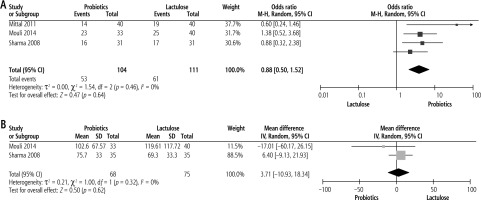
Probiotics compared with lactulose
Effect on reversal of MHE
Three studies compared the efficacy of probiotics with lactulose on reversal of MHE with pooled analysis showing lactulose to be more effective but not significant compared to probiotics with pooled OR of 0.88 (p = 0.64, 95% CI: 0.50 to 1.52) (Fig. 3A).
Effect on reduction of serum ammonia levels
There was no significant difference between the probiotics and lactulose groups in the lowering of serum ammonia levels based on pooled analysis from two studies. The pooled mean difference was 3.71 (p = 0.62, 95% CI: –10.93 to 18.34) (Fig. 3B).
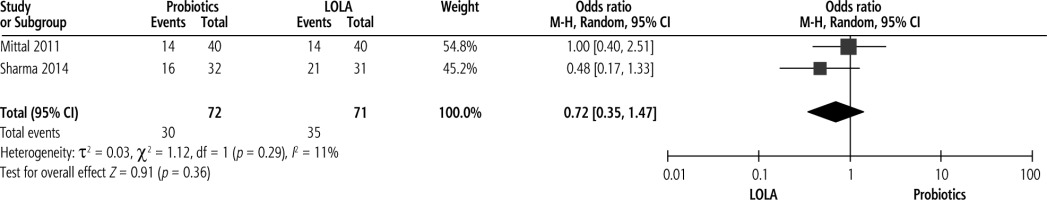
Probiotics compared with LOLA
Effect on reversal of MHE
Two studies compared efficacy of probiotics with LOLA on reversal of MHE with pooled analysis revealing no significant improvement of MHE with probiotics compared to LOLA, with pooled OR of 0.72 (p = 0.36, 95% CI: 0.35 to 1.47) (Fig. 4).
Probiotics compared with rifaximin
We found only one study that compared probiotics with rifaximin; thus we cannot perform a meta- analysis on this comparison. That study showed that 50% of patients (16 out of 32) improved after being treated with probiotics for two months, compared with 70.9% of patients (22 out of 31) who improved after being treated with rifaximin for the same period [17].
Discussion
The occurrence of hepatocellular failure, portosystemic shunting, or both, is characteristic of HE [24]. The severity of symptoms in HE influences the prognosis and treatment outcomes of the disease. MHE is the mildest form of disease onset and is distinguished by minor neurophysiological changes in cognitive function that are recognized by psychometric testing in the lack of clinical signs of mental alterations [25]. The psychometric hepatic encephalopathy score (PHES) is the test of choice for detecting MHE [26].
Ammonia is the neurotoxin that has been studied and discussed the most over the past century to elucidate neuropsychiatric phenotypes in liver cirrhosis patients [5]. Ammonia is an indispensable intermediate product produced by bacterial metabolism of amino acids and purines ingested by humans.
Under physiological conditions, approximately 90% of ammonia is excreted, predominantly by the synthesis of urea in the liver, the kidneys, and to a lesser extent by the muscle. In patients with cirrhosis, the decline in hepatocellular function and the presence of portosystemic shunting contribute to increased serum levels of ammonia.
When ammonia crosses the blood-brain barrier, it is processed in the astrocytes by glutamine synthase, transforming ammonia and glutamate into glutamine. Elevated glutamine in astrocytes contributes to the brain dysfunction observed in HE by causing an osmotic gradient, promoting water shift into astrocytes, resulting in edema, and generating reactive oxygen species [3].
In the current study, we present a meta-analysis of the effect of probiotics on 776 MHE patients from nine studies. Compared to placebo or no treatment, probiotics have been demonstrated to improve MHE; moreover, probiotics significantly reversed the MHE. There were also reductions in serum ammonia after probiotics treatment. This was in line with a previous meta-analysis undertaken by Cao et al. showing that probiotics are effective in MHE treatment, and better than placebo in reducing NCT, preventing OHE, and improving MHE [10]. More recently, Dhiman et al. also found that probiotics effectively reversed MHE and prevented episodes of OHE compared to placebo or no treatment [9].
When compared to lactulose, a meta-analysis from the current study showed lactulose to be more effective even though not significant compared to probiotics in reversing MHE. There was also no significant difference in the reduction of serum ammonia between probiotics and lactulose. This finding was similar to the results of a meta-analysis by Saab et al., indicating that the improvement of MHE was not significantly different between probiotics and lactulose [11]. Cao et al. also found that both probiotics and lactulose tend to improve MHE and reduce serum ammonia levels but there was no statistically significant difference between the modalities. Their study concluded that probiotics were essentially equivalent to lactulose therapy in their effectiveness in lowering serum ammonia levels and enhancing MHE [10].
A similar result was also found when comparing probiotics with LOLA. We found no significant difference in the reversal of MHE between probiotics and LOLA. This result is only based on two studies; thus further studies may be needed to provide more evidence on which treatment is better.
We could not perform a meta-analysis comparing probiotics and rifaximin, because we found only one study that compared these two modalities. In that study, rifaximin was superior to probiotics in improving MHE, with 70.9% of patients in the rifaximin group showing improvement compared with 50% of patients in the probiotics group. We thought that rifaximin, as the second most commonly used treatment of HE after lactulose, needs to be studied more in comparison with probiotics.
A small number of clinical studies on probiotics have yielded favorable findings regarding primary prevention of HE, risk of hospitalization due to HE, and severity of liver disease [6, 7]. The most common probiotic strains are lactic acid bacteria such as Lactobacillus, Lactococcus lactis, Streptococcus, and Bifidobacterium [27]. Lactic acid was thought to play a role in inhibiting bacterial urease activity that led to a decreased amount of ammonia in portal blood. Probiotics also reduce hepatocyte inflammation and oxidative stress, which in turn increase hepatic clearance of ammonia. In addition, probiotics can decrease the amount of ammonia due to its role in decrease intestinal permeability and ammonia absorption, enhance hepatic clearance of ammonia and other toxins, enhance the integrity of the intestinal epithelium, as well as strengthen immunity against urease or other bacteria that produce toxins. All these result in the improvement of MHE [10, 27].
Using probiotics (a combination of Clostridium butyricum and Bifidobacterium infantis) in MHE reduced harmful Enterococcus and Enterobacteriaceae in the gut microbiota. Blood levels of markers of bacterial translocation (lipopolysaccharide), intestinal permeability (D-lactate), and intestinal epithelial damage (diamine oxidase) were also decreased [15]. The consumption of the probiotic drink Yakult 400 reduced the quantity of Enterobacteriaceae in the gut microbiome [28]. In another RCT, administration of Lactobacillus GG for 8 weeks raised the number of beneficial bacteria (Lachnospiraceae and Clostridia XIV) and lowered the number of pathogenic bacteria (Enterobacteriaceae). This was also accompanied by a reduction in endotoxemia and systemic inflammation [20].
Although similar meta-analyses have been published, our study has some added value. One meta- analysis only compared probiotics to lactulose, whereas we did not limit the type of therapy we included to be compared with probiotics as long as the RCTs that compared them met our inclusion criteria.
The most recent meta-analysis before this study included RCTs only up to 2015 [9]. In that study, agents found to be the most effective in the reversal of MHE were rifaximin and lactulose, with probiotics also showing efficacy but not superiority compared to rifaximin and lactulose. This is similar to our finding that although probiotics are superior to placebo in reversing MHE and reducing serum ammonia, when compared to lactulose there was no significant difference in efficacy. In our study, we also included the most recent RCTs that had not been included in previous meta-analyses, adding novelty to our study.
However, there are several limitations to our study. First, most studies have intervention durations of less than three months. It takes time for probiotics to colonize and proliferate. Short term treatment may underestimate their true efficacy. Second, there is wide variability in the type of probiotics and doses used in the studies we included in the meta-analysis. Hence, it is unclear which type and dose of probiotics was the most efficacious.
Conclusions
The results of the present meta-analysis indicate that probiotics are an effective treatment for patients with MHE. Compared to placebo or no treatment, probiotics significantly reverse MHE and reduce serum ammonia levels. However, when compared to lactulose and LOLA, there was no significant difference in the reversal of MHE as well as reduction of serum ammonia levels.
We concluded that probiotics are equally effective as lactulose for MHE patients, but still cannot replace lactulose as a first line modality for MHE. Therefore, probiotics are a feasible choice for patients with poor tolerance to lactulose therapy.