Introduction
Crohn’s disease (CD) is a chronic autoimmune disease of the digestive tract, with poorly understood aetiopathogenesis. In the last 20 years there has been a significant increase in the incidence of inflammatory bowel disease (IBD) [1, 2]. During exacerbation, mainly steroids are used, which do not allow achievement of long-term remission, often causing dependence and significant side effects (SE) [3].
The biological therapy of CD patients is mainly based on tumour necrosis factor-α monoclonal antibodies (anti-TNF-α). In Poland, available preparations include infliximab (IFX), which is a human-mouse antibody and adalimumab (ADA), a recombinant antibody [4, 5]. Biological treatment represents a significant advance in the treatment of CD patients. These drugs reduce the number of surgical interventions and lead to endoscopic remission of the disease [6–8].
The guidelines do not regulate when and in which patients anti-TNF-α therapy might be stopped, due to insufficient literature and divergent results [9, 10]. The Polish National Insurance Office currently funds IFX therapy in CD patients for up to 2 years and ADA up to 1 year.
Aim
The aim of the study was to estimate the effectiveness and SE of the anti-TNF-α Polish therapeutic program in CD patients.
Material and methods
In this retrospective study the medical documentation was analysed of CD patients treated at the Department of Digestive Tract Diseases, Medical University of Lodz, Poland in the period from May 2008 to August 2017. In these patients, anti-TNF-α therapy (IFX or ADA) was administered for 1 year, in accordance with the characteristics of the medical product. The dose of the drug was not increased during the therapy period.
Enrolment criteria to biological therapy were: severe course of the disease (disease activity expressed according to Crohn’s Disease Activity Index (CDAI) > 300), the presence of fistulas, ineffectiveness, intolerance, or contraindications to immunomodulatory drugs and steroids.
Data were collected on the duration of therapy, SE, concomitant drugs, and the course of the disease: previous operations, complications of the disease (fistulas, arthritis, erythema nodosum) and the disease duration. Six and 12 months after therapy termination, the disease activity was assessed based on the information contained in the medical documentation: surgical interventions, steroids, immunomodulatory drugs, and biological therapies. The study received a positive opinion from the local Bioethics Commission (RNN/372/18/KE).
Statistical analysis
The statistical analysis was performed with Statistica 13.1 software. The Mann-Whitney U test was used for quantitative variables, and the χ2 test was used for nominal variables. Correlations between variables were examined using the Pearson test. Additionally, the Kaplan-Meier estimator was used in the analysis. The level of statistical significance was p < 0.05.
Results
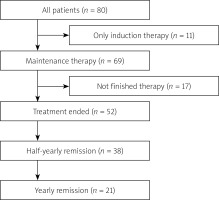
The medical histories of 80 CD patients (37 women and 43 men) treated with anti-TNF-α in the Department of Digestive Tract Diseases were analysed. The median age at the start of therapy was 31.5 (IQR: 24–40) years (Figure 1). Forty-two patients (52.50%) received IFX and 38 (47.50%) recieved ADA. In 11 (13.75%) patients only induction therapy was administered due to the lack of response to the treatment and severe respiratory infection (one case).
Maintenance therapy was not completed by 17 patients (seven treated with ADA and 10 with IFX). The most common cause of premature termination of therapy was disease exacerbation (6 cases, therein: two surgical intervention and 2 patients with abscess formation, in 2 patients the method of pharmacological treatment was changed). In addition, severe respiratory infections (5 cases), in 1 patient –- Clostridium difficile infection and in 1 patient – pregnancy. In four patients, data about the reason for early termination of therapy was unavailable.
Sixteen (20%) patients had experienced SE such as infections of the respiratory tract (62.50%), shingles, or mononucleosis (one case of each). SE occurred with a similar frequency in patients treated with ADA and with IFX (8/38 vs. 8/42, p = 0.823). Seven patients (8.75% of all patients) stopped therapy due to SE.
The remission time of 52 CD patients who underwent induction therapy and 1-year maintenance was analysed. Demographic data and clinical characteristics of patients are presented in Table I. Thirty (57.69%) patients used immunomodulatory drugs in addition to biological therapy. History of surgical operations before biological therapy concerned 16 (30.77%) patients. There was no significant difference between patients treated with IFX and ADA regarding age at start of therapy, age at diagnosis, gender, or additional use of immunomodulators.
Table I
Baseline characteristics of patients included in the analysis along with the division into patients receiving ADA or IFX
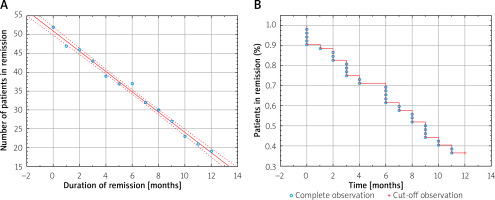
Immediately after 1-year maintenance therapy, 47 (90.38%) patients were in remission, after 6 months – 38 (73.08%), and after 12 months – 21 (40.38%) (Figure 1). A strong negative relationship between the number of patients in remission of the disease and the time (months) from the end of therapy (r = –0.996, p < 0.001) was found (Figure 2 A). After 9 months, only half of the patients maintained in remission as shown by the Kaplan-Meier estimator (Figure 2 B). Twenty (38.46%) patients were re-enrolled in the biological therapy program for 1 year, representing 64.52% of patients, who did not maintain annual remission following the end of therapy (the median time to next therapy: 231 days IQR:126.5–300.5).
Figure 2
A – Negative correlation between the time from the end of therapy and the number of patients in remission (r = –0.9958, p < 0.001). B – Kaplan-Meier curves for remission of Crohn's disease
Fifty-two (65%) patients completed the entire planned treatment period without serious SE, additional hospitalisations, and surgical procedures.
Discussion
Anti-TNF-α therapy effectively introduces patients to remission; however, after the end of treatment, the condition of a large number of patients worsens. It has been shown that the annual risk of exacerbation from discontinuing the use of biological therapy is 59.62% (95% CI: 41.52–61.44); the number of patients in remission decreased in a linear manner. In the meta-analysis by Kennedy et al. this risk was estimated at 36% (95% CI: 29–44%) [11]. Waugh et al. observed the incidence of disease relapse for 7 years after stopping treatment with IFX. Half of the patients suffered from the disease exacerbation after 16 months [12]. In our study the same rate was achieved after only 9 months, and patients were treated for the same period of time. Another study has shown that half of the CD patients were in remission 17 months after the end of therapy [13]. The differences could be caused by another definition of remission (clinical/endoscopic, with or without the use of immunomodulators). The duration of therapy was also different – in one study it lasted from 1 to 67 months [12]. In another meta-analysis, in which patients with CD were treated with IFX for at least 1 year, the annual risk of recurrence was similar to that shown in our study (43.9 ±5.0%) [14].
The number of patients in remission decreased during the year after the end of therapy by 24.62% in a linear manner. In other studies, in which maintenance therapy was continued for 1 year, the number of patients in remission decreased by only 6–9% [15, 16]. This indicates the need for longer maintenance therapy, at least in selected patients whose prognosis is worse.
We showed that up to 38.46% of patients within 1 year of the end of therapy were again included in anti-TNF-α induction therapy. It should be examined whether this approach affects the quality of life of patients and SE. An important aspect in biological re-treatment is the concentration of anti-TNF-α antibodies that may be responsible for poor reaction to treatment and hypersensitivity. A decrease in IFX concentration is associated with a high level of these antibodies [17]. However, the research is contradictory regarding whether its level lasts for many years or falls to an undetectable level [18, 19]. Studies have shown that up to 90% of patients respond well to re-treatment. This may be related to the satisfactory response of these patients to the previous therapy and the lack of hypersensitivity to these drugs [17].
From 2017, the Polish biological treatment program for CD patients allows the duration of IFX therapy to be up to 24 months. It will be interesting to see whether the extended treatment time affects long-term remission.
Approximately 9% of patients stopped therapy due to SE. Similarly, in another study IFX treatment was stopped in 11% of respondents due to SE [18]. Lehtola et al. showed that 21% of respondents had SE during 2 years of treatment and stopped the therapy for this reason [19]. The small differences are probably due to the longer duration of treatment in the described studies.
Anti-TNF-α therapy for patients with CD is relatively safe and effective. After 1 year of biological therapy exacerbations follow, and resuming the treatment is often necessary. A restricted time period of the therapy may contribute to further exacerbate the disease and entails the need to reassume biological therapy. Longer maintenance biological therapy in CD should be considered.