Summary
This study investigates the role of oxidative and antioxidant biomarkers in the pathogenesis of isolated coronary artery ectasia (CAE). It found significantly elevated levels of total oxidative status (TOS), oxidative stress index, and lipid hydroperoxide in CAE patients, while antioxidant markers remained unchanged. The findings suggest that increased pro-oxidant activity could play a critical role in CAE development. Smoking, TOS, and high-sensitivity C-reactive protein were identified as independent predictors of CAE. These results highlight the potential importance of therapies targeting oxidative imbalance in managing CAE.
Introduction
Coronary artery ectasia (CAE) is a distinct vascular anomaly characterized by localized or diffuse dilation of the coronary arteries, often linked to chronic inflammation and endothelial dysfunction. Patients with CAE are at increased cardiovascular risk, exhibiting symptoms ranging from stable angina to acute coronary syndromes, including myocardial infarction and sudden cardiac death [1–4]. While its pathophysiological mechanisms remain unclear, oxidative stress is a key driver of vascular inflammation and plays a significant role in arterial wall remodeling [5, 6].
CAE shares several pathological and clinical characteristics with coronary artery disease (CAD). Both conditions can lead to ischemic heart disease and myocardial infarction, although CAE can cause these complications in the absence of CAD through mechanisms such as sluggish flow, thrombus formation, and vasospasm. Histopathological studies have shown that CAE and atherosclerosis exhibit similar vascular changes and CAD frequently coexists with CAE, with up to 70% of CAE cases accompanied by atherosclerosis [1–3, 7]. Furthermore, oxidative stress has been extensively studied as a contributor to CAD, supporting its potential role in CAE [6].
Given the overlap between CAE and CAD and the established link between oxidative stress and CAD, investigating oxidative stress in CAE may provide valuable insights into its pathogenesis. However, limited studies have evaluated this association, particularly in isolated CAE (CAE without concurrent CAD) [8–10]. By focusing on patients with isolated CAE, this study seeks to clarify the independent role of oxidative stress in CAE.
Aim
To this end, we aimed to explore the association between oxidative stress and isolated coronary artery ectasia by examining oxidative and antioxidant biomarker profiles.
Material and methods
This prospective study was conducted at Mehmet Akif İnan Training and Research Hospital between January and September 2019. A total of 4356 patients who underwent coronary angiography were screened for the presence of CAE. Among these, 185 patients were identified with CAE. After applying exclusion criteria including significant coronary artery stenosis, history of acute coronary syndrome, left ventricular dysfunction, systemic inflammatory diseases, malignancy, and previous coronary interventions, 48 patients with isolated CAE were included in the study. The control group consisted of 32 age- and gender-matched individuals with normal coronary angiograms. The study was approved by the local Ethics Committee (Ref. 74059997.050.01.04/107). Informed consent was taken from all participants.
All participants underwent a comprehensive medical assessment, which included clinical history, physical examination, routine blood analysis, lipid profiling, electrocardiography, and transthoracic echocardiography. Hypertension (HT) was defined as systolic blood pressure (SBP) ≥ 140 mm Hg and/or diastolic blood pressure (DBP) ≥ 90 mm Hg on multiple measurements, or the use of antihypertensive medication. Diabetes mellitus (DM) was defined as fasting glucose levels ≥ 126 mg/dl, or the use of antidiabetic medications. Hypercholesterolemia was considered as total cholesterol > 200 mg/dl.
Coronary angiography was performed using the Judkin’s technique, with six French catheters introduced via femoral or radial arteries. Angiographic evaluations were conducted by two independent cardiologists blinded to patient data. CAE was diagnosed if the diameter of the dilated coronary artery segment was at least 1.5 times that of the adjacent normal segment, in the absence of significant coronary stenosis. Ectasia severity was categorized according to the Markis classification into four types: diffuse ectasia in two or three vessels (type 1), diffuse ectasia in one vessel with localized disease in another vessel (type 2), diffuse ectasia in a single vessel (type 3), and segmental localized ectasia (type 4) [11].
Oxidative and antioxidant biomarker analysis
Venous blood samples were taken prior to sheath removal during routine evaluations conducted before coronary angiography. Samples were centrifuged at 5000 rpm for 5 min, and the serum was separated and stored at –80°C until analysis. Oxidative and antioxidant biomarkers were measured using established methods:
Total antioxidant status (TAS) and total oxidative status (TOS) were measured using automated techniques developed by Erel [12, 13].
Oxidative stress index (OSI) was calculated as the ratio of TOS to TAC.
Lipid hydroperoxide (LOOH) levels were determined via the ferric ion oxidation-xylenol orange (FOX-2) method in an acidic medium [14].
Ceruloplasmin (CP) levels were measured colorimetrically using an automated method based on enzymatic oxidation [15].
Sulfhydryl (SH) groups were quantified using protocols described by Ellman and Hu [16, 17].
Paraoxonase-1 (PON1) activity was measured spectrophotometrically at 412 nm using paraoxon as the substrate [18].
Statistical analysis
Data analysis was performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). The Shapiro-Wilk test was used to evaluate the normality of continuous variable distributions. Descriptive statistics were expressed as mean ± standard deviation for normally distributed variables, or median and range for non-normally distributed variables.
Independent samples t-tests or Mann-Whitney U tests were applied for group comparisons based on data distribution. Correlations between variables were assessed using Pearson or Spearman correlation coefficients. Logistic regression analysis was employed to identify independent predictors of CAE, with variables showing p < 0.1 in univariate analysis included in the multivariate model.
Receiver operating characteristic (ROC) curve analysis was performed to determine sensitivity, specificity, and optimal cut-off values for biomarkers with significant differences between groups. A p-value < 0.05 was considered statistically significant.
Results
The study population included 48 patients with isolated CAE and 32 controls. Demographic and clinical characteristics showed no significant differences between groups in terms of age, gender, body mass index, or comorbidities such as hypertension and diabetes mellitus (p > 0.05 for all) (Table I).
Table I
Clinical and laboratory characteristics of the study population
Patients with CAE exhibited significantly higher levels of oxidative stress markers compared to controls: TOS: 30.14 ±8.81 vs. 23.88 ±4.74 mmol H2O2 equiv./l (p = 0.004), OSI: 3.21 ±1.12 vs. 2.43 ±0.53 arbitrary units (p < 0.001), LOOH: 11.95 ±2.88 vs. 10.13 ±1.66 µmol H2O2 equiv./l (p = 0.003). There was no significant difference in TAS, SH, CP and PON1 levels between the two groups (p > 0.05 for all) (Table II).
Table II
Oxidative/anti-oxidative biomarker levels of the study population
| Parameter | Isolated CAE (n = 48) | Control (n = 32) | P-value |
|---|---|---|---|
| TOS [mmol H2O2 equiv./l] | 30.14 ±8.81 | 23.88 ±4.74 | 0.004* |
| TAS [mmol Trolox equiv./l] | 1.06 ±0.22 | 0.98 ±0.09 | 0.14* |
| OSI (arbitrary unit) | 3.21 ±1.12 | 2.43 ±0.53 | < 0.001* |
| PON1 [U/l] | 75.74 [36.55–107.85] | 76.61 [41.1–103.4] | 0.56** |
| LOOH [µmol H2O2 equiv./l] | 11.95 ±2.88 | 10.13 ±1.66 | 0.003* |
| SH [mmol/l] | 0.35 ±0.21 | 0.37 ±0.12 | 0.45* |
| CP [U/l] | 485.33 ±75.31 | 476.12 ±63.85 | 0.54* |
Ectasia predominantly involved the right coronary artery in 29 (62%) cases, followed by the left anterior descending artery in 21 (45%) cases and the left circumflex artery in 18 (38%) cases. Based on the Markis classification, the distribution of CAE severity was as follows: type 1: 10 (20%) cases, type 2: 9 (19%) cases, type 3: 13 (27%) cases, type 4: 16 (34%) cases. No significant relationship was observed between antioxidant biomarker levels and the number or distribution of ectatic vessels.
Univariate and multivariate logistic regression analysis results are presented in Table III. Univariate analysis identified several factors significantly associated with the presence of CAE, including age (OR = 1.02, 95% CI: 1.00–1.03, p = 0.03), smoking (OR = 1.52, 95% CI: 1.01–1.92, p = 0.01), and oxidative stress markers such as TOS (OR = 1.02, 95% CI: 1.00–1.08, p < 0.001), OSI (OR = 1.11, 95% CI: 1.09–1.38, p = 0.01), and LOOH (OR = 1.12, 95% CI: 1.00–1.45, p = 0.02). In addition, high-sensitivity C-reactive protein (hsCRP) (OR = 1.21, 95% CI: 1.07–1.75, p < 0.001) was found to be significantly associated. In multivariate logistic regression analysis, smoking (OR = 1.85, 95% CI: 1.02–2.25, p = 0.02), TOS (OR = 1.05, 95% CI: 1.02–1.25, p = 0.01), and hsCRP (OR = 1.90, 95% CI: 1.46–4.52, p = 0.003) remained independent predictors of CAE.
Table III
Univariate and multivariate logistic regression analysis results
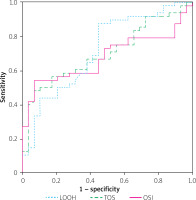
ROC analysis was performed to evaluate the diagnostic utility of oxidative stress biomarkers for identifying CAE and are shown in Figure 1. LOOH showed the highest diagnostic accuracy, with an area under the curve (AUC) of 0.71 (95% CI: 0.59–0.83, p < 0.001) and optimal cut-off value of 9.52 µmol H2O2 equiv./l (sensitivity: 87.5%, specificity: 55.2%). TOS had an AUC of 0.70 (95% CI: 0.58–0.81, p < 0.001) with an optimal cut-off of 24.70 mmol H2O2 equiv./l (sensitivity: 66.7%, specificity: 62.1%), while OSI achieved an AUC of 0.67 (95% CI: 0.56–0.79, p = 0.003) with an optimal cut-off of 2.35 arbitrary units (sensitivity: 72.9%, specificity: 51.7%).
Discussion
The findings of this study suggest that oxidative stress could play a significant role in the pathophysiology of CAE. Elevated levels of TOS, OSI, and LOOH in CAE patients indicate an increased oxidant burden in this population. These biomarkers collectively reflect the imbalance in oxidative stress and highlight the predominance of pro-oxidant activity in CAE. In contrast, antioxidant markers such as TAS, PON1, CP, and SH groups did not show significant differences between CAE patients and controls. This discrepancy suggests that the heightened oxidant load does not sufficiently trigger compensatory antioxidant mechanisms, resulting in a disruption of the oxidant/antioxidant balance in favor of oxidative stress.
The role of oxidative stress in CAE is supported by our comprehensive biochemistry panel, which included seven oxidative and antioxidant biomarkers. To our knowledge, this study is the first to evaluate the levels of LOOH, CP and SH groups in CAE patients. These novel findings could provide important insights into the oxidative and antioxidant dynamics in this disease.
The pathophysiological mechanisms underlying CAE remain incompletely understood, but several hypotheses have been proposed. Vascular inflammation, endothelial dysfunction and oxidative stress are considered central to its development. Chronic inflammation leads to degradation of the arterial extracellular matrix, particularly elastin and collagen fibers, resulting in weakening of the vessel wall and subsequent ectasia. Inflammatory cytokines and matrix metalloproteinases (MMPs) have been shown to play a key role in this process [1, 3, 7, 19]. Oxidative stress exacerbates these mechanisms by increasing the production of reactive oxygen species (ROS), which further activate MMPs and promote endothelial dysfunction. Endothelial dysfunction, reduced nitric oxide bioavailability, and increased vascular permeability are also implicated in the pathogenesis of CAE 6, 20, 21]. Together, these processes suggest that CAE is driven by a complex interplay of inflammation, oxidative stress, and structural vascular changes [1, 3, 4, 6–8].
The role of LOOH, a toxic byproduct of lipid peroxidation, is particularly noteworthy [14]. LOOH levels were significantly higher in our CAE patients compared to controls, underscoring its contribution to oxidative injury and vascular remodeling. CAE and coronary slow flow (CSF) share several overlapping mechanisms involving endothelial dysfunction, vascular inflammation and oxidative stress [22]. In our previous study on CSF, we observed similar findings, indicative of heightened oxidative activity in the pathophysiology of CSF and CAE [5]. Nevertheless, whether oxidative stress is a primary cause of CAE or a secondary effect remains unclear and warrants further investigation.
TOS serves as a comprehensive measure of overall oxidant activity in the body, and its significant elevation in our CAE patients aligns with findings in other vascular diseases, such as CAD and CSF 5, 8, 23]. In contrast, TAS did not differ significantly between CAE patients and controls, suggesting that the impaired antioxidant defense may not be the primary driver but rather an insufficient response to oxidative injury in CAE. The OSI, calculated as the ratio of TOS to TAS, provides a comprehensive measure of oxidative stress by balancing pro-oxidant and antioxidant levels [13]. The significantly higher OSI in CAE patients also underscores an overwhelming oxidative burden that surpasses the compensatory capacity of antioxidant defenses. Elevated OSI has been reported in other cardiovascular conditions, emphasizing its relevance as a marker of oxidative stress-mediated vascular damage 5, 8, 24].
PON1 is an antioxidant enzyme linked to high-density lipoprotein (HDL) cholesterol, preventing lipoprotein oxidation by hydrolyzing lipid peroxides. Reduced PON1 activity has been reported in conditions such as atherosclerosis, myocardial infarction, coronary artery ectasia hypercholesterolemia, and diabetes mellitus [25, 26]. However, our study found no significant difference in PON1 activity between CAE patients and controls, suggesting its limited role in CAE’s oxidative stress profile. Similarly, antioxidant systems such as CP and SH showed no significant differences between groups [27, 28]. These findings collectively indicate that the oxidative stress observed in CAE is primarily driven by increased pro-oxidant activity (TOS, LOOH, OSI) rather than diminished antioxidant capacity (TAS, PON1, CP, SH).
The interplay between oxidative stress and inflammation also warrants attention. Elevated hsCRP levels, an independent predictor of CAE in this study, suggest that oxidative stress may act synergistically with inflammatory pathways to drive vascular remodeling. ROS generated during oxidative stress can amplify inflammatory signaling by activating nuclear factor-κB (NF-κB) and upregulating pro-inflammatory cytokines, creating a vicious cycle of inflammation and oxidative damage.
Limitations. The main limitation of this study is the relatively small sample size, which may have limited our ability to detect significant differences in some antioxidant biomarkers. Additionally, the cross-sectional study design rules out establishing a clear causal link between oxidative stress and the development of CAE. Another constraint is that normal coronary arteries in the control group were identified using conventional angiography rather than advanced imaging techniques such as optical coherence tomography or intravascular ultrasound. This approach may have introduced potential bias in classifying the study population.
Conclusions
In our study, elevated levels of oxidative stress biomarkers, including TOS, OSI, and LOOH, were significantly associated with the presence of CAE. In contrast, antioxidant biomarkers showed no significant differences, suggesting that oxidative imbalance is primarily due to heightened pro-oxidant activity. These findings suggest that therapies targeting oxidative stress may offer potential benefits in managing this condition.