Introduction
Gastric hyperplastic polyps (GHPs) represent 30% to 93% of all gastric epithelial polyps [1]. They are characterized by dilated, elongated, tortuous foveolae (which impart the corkscrew appearance) lined by hyperplastic gastric mucin-containing epithelium [1]. Both the epithelium and mesenchymal stromal cells can show marked regenerative changes. Many factors such as Helicobacter pylori infection, chronic atrophic gastritis, portal hypertension, autoimmune gastritis and gastric surgery have been defined as risk factors for GHPs [2]. Most hyperplastic gastric polyps occur as single lesions; however, multiple polyps may also occur in 20% of cases [3]. The size of GHPs vary from a few millimeters to several centimeters [3]. They can occur in different parts of the stomach, including the body, fundus and gastroenteric anastomoses with or without antrum involvement [4].
Gastric hyperplastic polyps were noted in almost 1% of cirrhotic patients undergoing routine esophagogastroduodenoscopy (EGD) and can cause upper gastrointestinal bleeding leading to anemia [5]. The term portal hypertensive polyps (PHPs) has been used to describe GHPs in the context of cirrhosis [6]. Endoscopically, GHPs might present as reddish, slender, tandem with an earthworm like swelling on the surface and a remarkable increase of surface capillary vessels [4, 7]. Dysplasia in GHPs may develop from them and are found in up to 19%, and adenocarcinoma has been reported in about 2.1% of resected polyps. The risk is strongly associated with the size, an observation that has led to the recommendation of removal of polyps larger than 5 mm or 10 mm [8].
The standard treatment for PHPs > 10 mm is polypectomy. Resection of GHPs in cirrhotic patients by polypectomy is associated with a high risk of the bleeding disorder referred to as coagulopathy, complicating hepatic dysfunction, and portal hypertension induced thrombocytopenia [2, 3].
Hot snare polypectomy (HSP) is the most acceptable procedure for HP resection despite the high rate of complications (bleeding and perforation) which have been reported in a range of 2.7% to 23.9% [8]. Although there are several preventive measures, post-polypectomy hemorrhage remains a major complication of polypectomy until now, especially in patients with liver cirrhosis [9].
Endoscopic band ligation (EBL) by suction equipment and rubber bands has been used to eradicate esophageal varices in patients with cirrhosis related portal hypertension [10].
In the literature, a few studies and one case report have evaluated EBL as proper management of GHPs even in presence of bleeding or bleeding liability [11–14].
In this study, we aimed to assess the efficacy of EBL for GHP resection in patients with liver cirrhosis.
Material and methods
Study design
This is a single-center prospective study carried out at the National Liver Institute (NLI), Menoufia University. Eligible patients aged ≥ 18 years were enrolled between October 2018 and October 2020.
Informed consent was taken and GHPs detected through EGD in cirrhotic patients were evaluated within the scope of our study. Findings such as esophageal and/or gastric varices, ascites, splenomegaly, and portal hypertensive gastropathy are diagnostic for the presence of portal hypertension [6]. Multiple bio-psies were taken from all polyps for histopathological assessment. The histological diagnostic criteria of GHPs were set as the presence of foveolar hyperplasia with an inflammatory and abundant chorion or long, deep, and hypersecreting crypts [7].
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of National Liver Institute, Menoufia University (NLI IRB OOOO3413 FWAOOOO227).
Demographic, clinical, and endoscopic variables
Demographic and clinicopathologic data were retrieved including age, gender, symptoms, etiology of liver disease, and CTP score of cirrhosis. Endoscopic data, i.e., GHP number, size, location, and morphology, were collected from EGD reports. Operation details including operating time, established blood loss, complications, interventions, number of sessions and recurrence were also recorded. Perforation or hemorrhage was considered as a complication of the procedure. The hospitalization expenses mainly consisted of the cost of the procedure and that of perioperative care. Upper endoscopy was repeated for all patients at 3-, 6- and 12-month post-treatment time points for recurrence.
All endoscopic resections were done by experienced endoscopists using the standard method. All patients were informed of the benefits and risks of the procedure. Eligible polyps were randomized (1 : 1) to be managed with either EBL or HSP.
EBL procedure
Endoscopic band ligation is done using a banding device attached to the tip of the endoscope. The polyp is sucked into the banding chamber, and a trip wire dislodges a rubber band ligating the entrapped polyp [4].
HSP procedure
Hot snare polypectomy is a minimally invasive endoscopic procedure used to remove polyps from the upper gastrointestinal tract. During the procedure, the endoscope is inserted through the mouth and into the esophagus and stomach. The camera on the end of the scope allows visualization of the polyp. A snare wire loop is passed through the endoscope and positioned around the base of the polyp and tightened, allowing it to be removed from the body. Cauterization is used to prevent bleeding and reduces the risk of complications.
Outcomes: The primary outcome of the current study was the efficacy of EBL maneuvers and secondary outcomes were complication rates, polyp retrieval rates, and procedure time.
Statistical analysis
Data were collected, tabulated, and analyzed statistically using an IBM personal computer with SPSS Statistics version 20 (IBM, Chicago, Illinois, USA). Quantitative data were presented as mean, standard deviation (SD), and range, and qualitative data were presented as numbers and percentages. The chi-square test (χ2) was used to study the association between two qualitative variables. The t-test was used for comparison between two groups normally distributed having quantitative variables and the Mann-Whitney test used for comparison between two groups not normally distributed having quantitative variables. The significance level was set at a p value of < 0.05.
Results
Among the 100 cirrhotic patients in the current study, 50 received EBL and 50 received HSP randomly (1 : 1). As shown in Table 1, the demographic and clinical characteristics of the patients were balanced between the two study groups. The mean age of the included patients was 57.5 ±6.26 years in the EBL group and 59.6 ±5.83 years in the HSP group, p value = 0.080. The mean polyp size for the included patients was 1.46 ±0.42 cm in the EBL group and 1.52 ±0.45 cm in the HSP group, p value = 0.518. The mean number of polyps was 2.44 ±0.91 in the EBL group versus 2.12 ±0.89 in the HSP group, p value = 0.092. There was no significant difference in the morphology (p value = 0.317) and location (p value = 0.369) between the two groups (Table 1).
Table 1
Demographic and clinical characteristics of patients
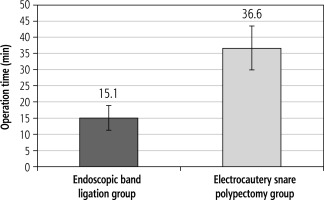
Endoscopic characteristics and clinical outcomes of patients treated with EBL or HSP (Table 2). The mean operating time was significantly shorter in patients who received EBL than patients received HSP (15.1 ±3.80 min vs. 36.6 ±6.72 min, p value < 0.001) (Fig. 1). Complications were significantly higher in the HSP group; 20% had bleeding (controlled by argon plasma coagulation (APC) in 8 patients and by combined APC and adrenaline injection in the other 2 patients) while no bleeding was reported in the EBL group (p = 0.003). The mean hospital cost was also significantly lower in the patients who received EBL (280.0 ±2.02 EGP) than those who received HSP (390.0 ±181.8 EGP), p value < 0.001. However, the recurrence in EBL and HSP patients during the follow-up period was 12% and 10%, respectively, with no significant difference (Table 2).
Table 2
Endoscopic characteristics and clinical outcomes of patients
Discussion
Gastric hyperplastic polyps are incidentally discovered during screening, prophylactic, and therapeutic EGD in patients with portal hypertensive cirrhosis [4]. In patients with liver cirrhosis, the extensive mucosal damage and repair, antral hypercontraction and hypergastrinemia caused by proton pump inhibitor (PPI) abuse also might add more complexity [10]. Notably, despite the questionable potential precancerous liability, GHPs should be resected whenever incidentally detected [8].
The high rates of bleeding, both quantitative and qualitative, associated with HSP (the standard management maneuver) might represent a major concern especially in patients with liver cirrhosis [15].
Only a few studies have tried to investigate other procedures rather than HSP for resecting HPs with documented lower complication rates [4, 15, 16]. Despite the devaluation, the EBL seemed to be the perfect option for patients with liver cirrhosis to be done with esophageal varices band ligation in the same setting.
The current study assessed the efficacy of EBL, compared to the standard HSP for resecting GHPs in cirrhotic patients. Remarkedly, EBL performed greatly when considering the time needed to complete the procedure, which was found to be significantly shorter than the time for HSP (15.1 ±3.80 min vs. 36.6 ±6.72 min, p value < 0.001).
The expertise of the performer along with the specialized centers of excellence might add more professionalism and less impediments. However, bleeding was reported to be highly associated with HSP (20%) compared to null cases with bleeding in the EBL group of patients. These estimated rates of bleeding with HSP reported in the literature ranged from 1.0% to 7.2% in most studies [17–20]. In the current study, the higher bleeding liability in cirrhotic patients is responsible for the high discrepancy rates when compared to other studies.
In snare polypectomy the cutting action of the snare loop may cause injury to blood vessels within or near the polyp base, leading to bleeding. Additionally, incomplete polyp removal [21], larger or more vascular polyps [22], and/or polyps located in rich blood supply areas were found to be associated with higher bleeding risk [23]. Lastly, the rare post-polypectomy syndrome characterized by abdominal pain, fever, and rectal bleeding occurring within 1-2 weeks after polypectomy is thought to be related to an inflammatory response following the procedure [24]. Occasionally, perforation of the bowel wall can occur during snare polyp resection and may result in significant bleeding [25]. Remarkedly, the risks of bleeding after a snare polypectomy are heightened in patients with chronic liver disease due to the accompanying thrombocytopenia, coagulation dysfunction, and portal hypertensive gastropathy with increased fragility of blood vessels [26]. However, these risks are minimized following polyp EBL even in patients with liver cirrhosis. The technique does not involve cutting or injuring blood vessels directly. The rubber band placement cuts off the blood supply gradually, at the polyp bases, which in a few minutes become congested and cyanotic with complete avascular necrosis leading to bloodless transection [27].
Additionally, the current study suggested that EBL is an effective modality in elective treatment of asymptomatic HPs as well as bleeding lesions. This is in accordance with a case report with bleeding GHP in a challenging uremic patient on antiplatelet therapy. A report documented the role of EBL as an efficacious measure in treating GHP in patients with high bleeding liability [28]. The advantages of EBL might include a bloodless biopsy even in patients with bleeding liabilities for avascular necrosis [12].
Moreover, patients with liver cirrhosis are always in need of upper endoscopy either for screening or therapeutic for esophageal varices allowing EBL for gastric polyps – if present – in the same session, conserving costs, time, and suffering.
The mean hospital cost was also significantly lower in the patients who received EBL than those who received HSP (280.0 ±2.02 vs. 390.0 ±181.8 EGP), p value < 0.001. Fruitfully, these reductions in the EBL cost might be more appropriate for patients with liver cirrhosis who were already financially exhausted.
The selection of the technique may be influenced by factors such as the polyp’s size and location, the presence of skilled professionals, and the availability of necessary equipment and expertise. EBL has shown better performance when dealing with different types of polyps (sessile or pedunculated), unlike HSP, which had a limited approach to the sessile polyps [28]. The EBL method allowed a lesion to be easily captured into the transparent hood, even in cases where it was situated tangentially and in sessile polyps.
Additionally, HSP is challenging when dealing with lesions in the lesser curvature, or the posterior wall and cardia of the stomach. On the other hand, EBL had a wide range of maneuverability whatever the lesion site [14].
Reportedly, EBL seems to be the ideal choice of bleeding GHP less than 1 cm in terms of technical difficulties, and HSP remains the only option for larger sizes [28].
However, the current study reports non-superiority of both maneuvers in treating GHP as regards size, number, or location.
To the best of our knowledge, this is the first study treating GHP in liver cirrhosis patients with high risk of bleeding by EBL. Notably, it proved efficacy, safety, shorter procedure time and lower cost, and added to the easy approach to any types of polyps. Advantageously, it can be done in the same setting with esophageal varices band ligation, which is done frequently in this category of patients, resulting in less suffering, lower costs, and higher safety. However, further validation of this maneuver in this critical cohort with large scale studies is still needed.