Introduction
Portal hypertension is a common complication of liver cirrhosis. Varices are dilated collaterals that develop as a result of portal hypertension (HTN) at the level of the porto-systemic connections and can cause a shift in the flow blood from high to low pressure. They are identified during endoscopy as dilated tortuous vessels with increased risk of bleeding. Common locations for porto-systemic shunts are the lower oesophagus and the gastric fundus [1].
Acute gastrointestinal bleeding in the setting of portal hypertension and liver cirrhosis is a medical emergency with mortality as high as 20% at 6 weeks [2]. Variceal bleeding is the aetiology behind upper GI bleeding in up to 70% of cases. The most common sites of acute variceal bleeding are oesophageal varices (OV) and gastric varices (GV) [3].
Ectopic varices (EcV) are defined as dilated tortuous veins located at unusual sites other than the gastro-oesophageal junction [4]. Ectopic varices may result secondarily to portal hypertension or segmental obstruction of one of the portal vein tributaries, which can result in segmental portal hypertension (for example, thrombosis of superior or inferior mesenteric veins or of splenic vein) [5].
Although not commonly seen, the misinterpretation and the mismanagement of ectopic varices may lead to major complications or even to death. Hence the importance of proper identification and treatment of this specific type of varix [6].
Local endoscopic therapeutic methods for variceal bleeding may include band ligation, injection sclerotherapy, and injection of tissue adhesive [7]. The recurrence rate after endoscopic therapy was documented to be high with the need for re-intervention in up to 30% of cases [8, 9].
The first step in management includes resuscitation by crystalloids and packed red blood cells (RBCs) with initiation of prophylactic antibiotics. Terlipressin and other vasoactive drugs can be used, despite no randomized control trials to recommend their use [10].
The second step includes emergency endoscopy after resuscitation if no obvious source is detected upon upper endoscopy; recto-sigmoidoscopy is done after rapid preparation [11]. The source of bleeding (spurter or white nipple) can be managed accordingly [12].
Failed endoscopic control of bleeding ectopic varices can be managed by interventional radiology techniques like trans-jugular intrahepatic porto-systemic shunt (TIPS) or balloon-occluded retrograde transvenous obliteration (BRTO) [13, 14].
Recently, endo-sonography has started to play an emerging role in the management of portal hypertension through the placement of vascular coils inside the lumen of bleeding varices, through the needle, guided by sonographic control. This can be followed by injection of cyanoacrylate [15].
Finally, surgery may be needed after failed endoscopic and radiological interventions, which may include dearterialization, stapling, surgical ligation, or shunt surgery. Only rarely will surgical intervention provide long-term management of bleeding. Creating surgical portosystemic shunts is rarely done today because it is associated with high morbidity and mortality, especially in patients with decompensated cirrhosis [16]. A number of consensus meetings have been organized as a result of recognizing the difficulty in the management of ectopic varices. Today their management is variable according to the protocol approved by each facility [17].
Aim
Our objective was to study the endoscopic assessment of ectopic varices as well as necessary haemostatic interventions to the best of our knowledge. We aimed to perform a review of the literature to compare our results to the latest available data.
Material and methods
Data collection
All patient data with regard to demographic characteristics (age and sex), endoscopic findings (presentation of the patients, aetiology, Child score, source of bleeding, site, and size of ectopic varices).
Methodology
This is a multi-centre retrospective study in 5 tertiary care endoscopic referral centres. Our group extracted endoscopic reports of patients presenting to the emergency department with evidence of recent GI bleeding in whom ectopic varices were identified. The research included patients presenting with evidence of active GI bleeding: haematemesis, melena, or fresh bleeding per rectum. After resuscitation and stabilization of the general condition, upper endoscopy or recto-sigmoidoscopy were performed to detect and treat the source of bleeding. We included patients in whom ectopic varices were identified during the procedure. We reported all interventions or procedures needed, details of hospitalization, radiological and laboratory results, as well as follow-up charts.
All included patients had written informed consent in their files. The researcher defined the inclusion criteria as follows: 1) Patients presenting with evidence of GI bleeding, 2) Presence of ectopic varices in the endoscopic report.
Patients were excluded if no informed consent for endoscopic examination was available. All procedures were performed by an experienced endoscopist with experience in more than more than 1000 interventional procedures, and all files and recorded images were revised by 2 other endoscopists to confirm the diagnosis and approve the plan of management.
Statistical analysis
The researcher analysed the difference in the distribution of frequencies among different groups using the χ2 test. For continuous variables, an independent t-test analysis was carried out to compare the means of normally distributed data. Median differences of the data that did not follow the normal distribution were calculated by the Mann-Whitney U test. Pearson’s correlation coefficient was used to study direct correlations between multiple variables. Data were analysed using the SPSS statistical software version 16 (SPSS Inc., Chicago, USA).
Results
We reviewed the endoscopy reports of 5458 patients presenting to the emergency department with GI bleeding in the period from January 2018 to December 2021 in 5 referral centres. Of these reports, we extracted 4689 upper endoscopy reports and 769 reports of recto-sigmoidoscopy. We imported only reports that identified the source of the bleeding (if the patient had more than one endoscopy report) (Figure 1).
Figure 1
Scheme of data extracted from emergency endoscopy database of patients presenting with evidence of acute GI bleeding included in the study
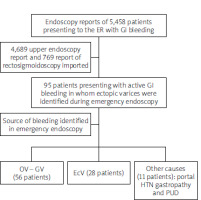
We included in our study 95 patients presenting with active GI bleeding in whom ectopic varices were identified during emergency endoscopy (within 24 h of admission). Direct endoscopic interventions were applied for all cases with active GI bleeding. The characteristics of patients included in our series are summarized in Table I. All patients presenting with active GI bleeding underwent endoscopic exploration and intervention according to the source of bleeding. The mean age was 57.3 ±9.4 years. There were 52 (54.7%) males and 43 (45.3%) females. The most common aetiology was liver cirrhosis in 71 (74.7 %) patients, followed by liver cirrhosis associated with hepatocellular carcinoma (HCC) in 15 (15.8%) patients. Less common causes of GI bleeding associated with ectopic varices were portal vein (PV) thrombosis and superior mesenteric vein (SMV) thrombosis, identified in 7 (7.4%) and 2 (2.1%) patients, respectively.
Table I
Sociodemographic characteristics of patients involved in the study, aetiology of the portal HTN, as well as their Child score and clinical presentation (number and percentage) (95 patients presenting with active GI bleeding, in whom ectopic varices were identified during emergency endoscopy)
In our series, all patients in whom ectopic varices were discovered during emergency endoscopy for GI bleeding had evidence of portal hypertension. Excluding 9 patients with PV and SMV thrombosis, all other patients had evidence of liver cirrhosis (86 patients). Their Child-Pugh score was distributed as follows: 30 (31.4%) patients had score A, 49 (48.8%) patients had score B, and 16 (19.8%) patients had score C. Melena was the most common presenting symptom in 46 (48.4%) patients, while haematemesis and fresh bleeding per rectum were less common – 26 (27.4%) cases and 23 (24.2%) cases, respectively.
Endoscopic interventions for haemostasis included 2 techniques: A) Intra-variceal injection of N-butyl-cyanoacrylate (Histoacryl®) – each injection was prepared using a mixture of 0.5 ml cyanoacrylate and 1 ml lipiodol (Guerbet Laboratory, Aulnay-Sous-Bris, France). The volume of tissue adhesive injected inside the varix was evaluated by the operator according to the size of the varix. B) Band ligation for ectopic varices performed using multiband devices (Wilson-Cook Medical, Winston-Salem, NC, USA).
All patients presenting with active GI bleeding from duodenal varices were treated by intra-variceal injection of N-butyl-cyanoacrylate as described [18]. For rectal varices, 8 patients were treated by application of an elastic band, and 1 patient was treated by injection of tissue adhesive on top of active spurter bleeding [19].
Regarding the rest of the patients, other aetiology for the GI bleeding was recognized and treated according to the approved guidelines. Oesophageal and gastric varices were the most common site of bleeding in 56 (58.9%) patients while portal hypotensive gastropathy and peptic ulcer disease were less common sources of bleeding in 9 (9.5%) and 2 (2.1%) patients, respectively (Table II).
Table II
Distribution of the source of bleeding in our cohort during emergency endoscopy
| Source of bleeding | Number of patients |
|---|---|
| OV-GV | 56 (58.9%) |
| Ectopic varices | 28 (29.5%) |
| Portal HTN gastropathy | 9 (9.5%) |
| Peptic ulcer disease | 2 (2.1%) |
In our study, the majority of ectopic varices discovered upon emergency endoscopy for GI bleeding were located in the rectum (49 patients). Duodenal varices were seen in 38 patients, sigmoid varices in 5 patients, and antral varices in 3 patients (Table III). In this group of patients, ectopic varices were attributed as the origin of bleeding in 28 patients. Bleeding from duodenal varices was found in 19 patients, and rectal varices in 9 patients.
Table III
Characteristics of ectopic varices identified during emergency endoscopy in terms of site and size of the varix
| Variable | Number of patients | |
|---|---|---|
| Site of ectopic varices: | ||
| Rectal | 49 (51.6%) | |
| Duodenal | 38 (40%) | |
| Sigmoid | 5 (5.3%) | |
| Antral | 3 (3.1%) | |
| Size of ectopic varices: | ||
| Large | 14 (14.7%) | |
| Medium | 28 (29.5%) | |
| Small | 53 (55.8%) | |
Ectopic varices were classified by the operating endoscopist according to their size as small, medium, and large. Small EcVs were seen in 53 (55.8%) patients, medium in 27 (29.5%) patients, and large varices were identified in 14 (14.7%) patients, from all patients included in the study.
Failure of endoscopic management was defined as evidence of rebleeding within 5 days of index endoscopy and the need to change the line of therapy according to the Baveno criteria [20].
Rebleeding from ectopic varices was found in 5 cases, for whom interventional radiology was performed (emergency TIPS). All cases with rebleeding were large duodenal varices previously treated by intra-variceal injection of N-butyl-cyanoacrylate. Rebleeding was controlled in 2 patients, and mortality occurred in 3 cases after re-intervention (hepatocellular decompensation).
Statistical analysis
Statistical analysis using Pearson’s correlation showed no causality relation between the age, gender, or site of the EcV and the risk of bleeding from ectopic varices in our study population. Positive relation was found between Child score (p = 1.070), HCC (p = 0.0036), PV thrombosis (p = 0.0677), and size of the EcV (p = 0.0001). However, these results could not be confirmed upon univariate and multivariate regressive analysis because the number of cases was not statistically sufficient. In our study, rectal varices were the most common site for ectopic varices in 49 (51.6%) patients. However, duodenal varices carry a higher risk of rebleeding and need for re-intervention.
Discussion
Ectopic varices are defined as complex vascular shunts that arise secondarily to portal hypertension outside common sites (lower oesophagus and gastric fundus) and can lead to gastro-intestinal bleeding [10].
Management of ectopic varices is challenging because of their great diversity and absence of clear consensus on proper direction of therapy (not based on the results of controlled trials) [12, 21].
Endoscopic therapy remains the cornerstone in the diagnosis and initial intervention for life-threatening GI bleeding [10]. Management of EcV may be different according to its presentation, location, and coexisting morbidities [7, 22].
The correct decision in such patients is individual to each case scenario and requires a multi-disciplinary approach including medical, endoscopic, radiological, and surgical interventions [23]. Due to scarcity of information related to EcV largely derived from single-centre case reports and review articles, more global and multicentre research efforts are still needed to highlight the clinical features, the outcome, and the risk factors related to bleeding EcV [24].
EcVs may present at the site of embryonic vascular channels or secondarily to reversal of flow in the veinous system. They can be classified according to their location as luminal and extraluminal [25].
Luminal ectopic varices can be located anywhere in the gut wall other than the gastro-oesophageal junction. Extraluminal ectopic varices may be located in the retroperitoneal, intraperitoneal, or periumbilical areas, in the gall bladder and biliary tree, at the ovary, or in the vagina [26].
Endoscopic treatment for ectopic varices requires a certain level of endoscopic experience because they are usually located in difficult sites of the gastrointestinal tract [27, 28]. In our study, we did not identify any patient with ectopic varices in more than one location (mixed sites).
Oesophageal and gastric varices are more studied than duodenal and rectal varices, with more guidelines supporting their treatment [24]. It is worth noting that EcVs are dangerous and are responsible for up to 5% of all variceal bleeding. Duodenal varices carry 4 times greater risk of bleeding compared to oesophageal varices [25].
In our study, we reported 3 different groups of bleeding ectopic varices as follows: 1) duodenal varices, 2) rectal varices and other eccentric sites (antrum, sigmoid colon).
The first group comprises duodenal varices, identified at the level of the first 2 segments of the duodenum during upper endoscopic inspection. Rectal varices were identified during urgent recto-sigmoidoscopy.
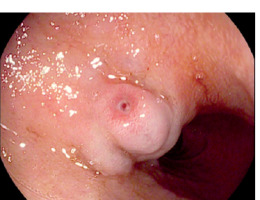
Duodenal varices represent about 40% of all ectopic varices identified in our series. They may originate from afferent vessels: the superior and inferior pancreatico-duodenal veins, cystic branches of the superior mesenteric veins, gastroduodenal vein, pyloric vein, the efferent veins of Retzius, or the inferior vena cava (Figure 2).
Figure 2
Upper endoscopic view of ectopic varices seen at the level of the bulb of the duodenum, showing white nipple sign
Rectal varices were more common in our study, representing about 52% of ectopic varices. They originate from afferent superior rectal veins to the efferent middle and inferior rectal veins, tributaries of internal iliac and pudendal veins (Figure 3).
Figure 3
View from rectosigmoidoscopy for a patient presenting with fresh bleeding per rectum, showing multiple rectal varices with ecchymosis and surface erythema
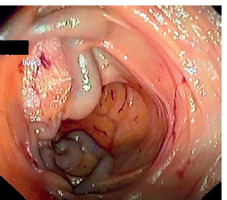
Rectal varices are differentiated from internal haemorrhoids by being dilated, tortuous, dark blue, and originating above the dentate line. They are compressible and refill immediately on release, and they do not prolapse into the proctoscope on examination.
The ideal treatment modality for EcV has not yet been identified. Although its efficacy and safety are still unknown, endoscopic treatment is currently the most popular therapeutic option for EcV bleeding because it is less invasive and technically less challenging than surgical and radiological interventions like the trans-jugular intrahepatic portal systemic shunt (TIPS) procedure and balloon-occluded retrograde transvenous obliteration (BRTO) [29, 30].
We identified 5 patients with early rebleeding, as shown by persistence of melena or bleeding per rectum 72 h after index endoscopy, or drop of haemoglobin despite blood transfusion.
All patients who experienced early rebleeding had large duodenal varices. Although emergency TIPS was performed, 3 patients died secondarily to hepatocellular decompensation. These patients shared the same profile of large duodenal varices in the setting of liver cirrhosis.
We noted in our study that duodenal varices carry a higher risk of rebleeding after initial endoscopic interventions, with higher mortality than other aetiologies and other types of ectopic varices. Our explanation of the higher risk of rebleeding from duodenal varices compared to other sites is the presence of a rich vascular network at that level compared to rectal varices. The number of patients was not appropriate for direct statistical correlation.
Conclusions
Management of bleeding from ectopic varices is difficult and depends on the location and initial presentation. Our study describes a series of patients with EcV discovered upon emergency endoscopy. Rectal varices were the most commonly found in our series. Bleeding and the need for re-intervention is more common in duodenal varices. Case-control studies are needed to provide clear guidelines for the best management of each type of ectopic varix.










