Glomus tumours (GT) are proliferative lesions originating from the mesenchymal tissue of glomus bodies, which are arteriovenous shunts involved in thermoregulation of the subungual zones of the fingers and toes [1, 2]. These tumours are rare, accounting for less than 2% of all soft-tissue tumours [2]. Glomus bodies are even rarer in the visceral organs, including the gastrointestinal tract [3]. Female predominance is seen in this tumour epidemiology, and it usually occurs in the fifth or sixth decade of life [1, 2].
The stomach is the most common site of GT in the gastrointestinal tract [4]. The most common presentation is gastrointestinal bleeding, although many cases might be discovered as an incidental finding during upper endoscopy. Endoscopically they are seen as subepithelial lesions, which may resemble carcinoid tumours or gastrointestinal stromal tumours (GIST) among others [5]. Immunohistochemistry (IHC) should be done for definite diagnosis of GT [2, 4]. Most gastric GT are benign, but rare events of malignant transformation have been reported [4]. Although duodenal GT have been reported in the past, they are considered very rare [6].
A hypertensive and otherwise healthy 57-year-old man presented with a history of recent-onset epigastric pain and post-prandial dyspepsia. Upper GI endoscopy revealed a round subepithelial lesion in the second part of the duodenum, and endoscopic ultrasound (EUS) showed the lesion in the second part of the duodenum, measuring about 12 × 7 mm with mixed echo appearance, originating from submucosa, with well-defined borders, and without penetrating to other layers. No surrounding lymph node was detected.
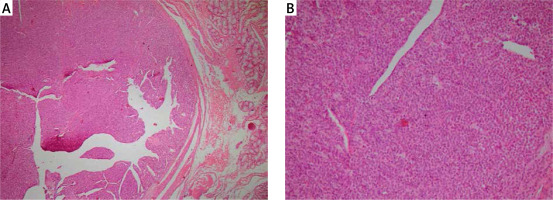
Endoscopic mucosal resection was done by applying a rubber band at the base of the lesion and resection with a hot snare. The resected tissue was retrieved with a basket. Histological evaluation of the lesion showed a round submucosal mass consisting of dilated blood vessels lined by normal endothelial cells and surrounded by a solid proliferation of round cells with perfectly round nuclei and acidophilic cytoplasm. There was no mitosis or atypia (Figure 1). Immunohistochemically, the duodenal tumour cells stained positive for smooth muscle actin (SMA) and vimentin and were negative for chromogranin, C-kit, desmin, cytokeratin, HMB45, Melan-A, and S100, with low proliferative index confirming the diagnosis of GT of the duodenum.
Figure 1
Histology of the resected lesion. A – Haematoxylin-eosin, original magnification 100×. B – Haematoxylin- eosin, original magnification 200×
Post-resection clinical and endoscopic evaluation at 6, 12, 24, and 48 months did not show any evidence of tumour recurrence.
The patient has provided informed consent for publication of the case.
This report describes a very rare case of duodenal GT presenting with dyspepsia. The diagnosis was confirmed after endoscopic resection with histology and IHC staining.
GT are uncommon soft-tissue tumours comprising less than 2% of all soft tissue neoplasms [2]. These tumours originate from smooth muscle cells of the glomus bodies, which are modified arterio-venous structures, which play a role in thermoregulation [7].
GT in the gastrointestinal tract is rare, and most cases are reported in the stomach [1, 2, 4]. Presenting symptoms are variable and include from total asymptomatic to upper or lower gastrointestinal bleeding, anaemia, epigastric discomfort, nausea, and vomiting [3, 4]. Duodenal GT may present similarly, with bleeding or signs of obstruction, or may be an incidental finding on endoscopy [8, 9].
Computed tomography and EUS may help in the characterisation of these lesions [5]. Gastrointestinal GT should be differentiated from other tumours, including GIST, carcinoid tumours, hemangiopericytomas, paragangliomas, and lymphomas, which may have similar appearance to GT [5].
There are no specific clinical and imaging findings for GT, which makes the definite diagnosis difficult. IHC is the most reliable diagnostic tool. Morphologically the cells are small, uniform, and round with surrounding capillaries [10]. On IHC they stain positive for SMA, vimentin, calponin, collagen type IV, and laminin [2].
Surgical treatment of gastrointestinal GT consists of wedge resection or excision of the tumour, depending on the location and size [10, 11]. There is now growing evidence that endoscopic resection of GT is possible, and in expert centres it would be a safe method. Endoscopic submucosal dissection is the preferred treatment in centres with the greatest experience in gastric GT [12]. In this case we used band ligation and a hot snare without injection to resect the lesion. This technique was reported to be safe for removal of lesions up to 20 mm from the gastrointestinal tract [13]. We retrieved the tissue with a basket and could have a complete resection confirmed with histologic examination. Four years after resection there was no recurrence on endoscopy or EUS.
In conclusion, GT of the duodenum should be considered in the differential diagnosis of subepithelial lesions in this region. They could be treated safely with endoscopic techniques.










