Summary
The best treatment option for acute uncomplicated type B aortic dissection is unclear. This meta-analysis compares the clinical efficacy and safety between thoracic endovascular aorta repair and best medical treatment used for treating acute uncomplicated type B aortic dissection. We found that thoracic endovascular aorta repair was associated with a better long-term patient prognosis than best medical treatment.
Introduction
Acute type B aortic dissection (TBAD) is an extremely dangerous and life-threatening condition [1–3], cases of which can be classified as complicated or uncomplicated [4]. Cases of complicated TBAD are characterized by instances of dissection that coincide with refractory pain, rapid aortic expansion, malperfusion syndromes, or rupture at time of onset or during hospitalization, whereas these conditions are absent in uncomplicated cases [4]. Thoracic endovascular aorta repair (TEVAR) is the most common strategy employed for the management of complicated TBAD cases [5], whereas best medical treatment (BMT) is frequently employed to care for patients affected by uncomplicated TBAD. At present, however, the BMT-based management of uncomplicated TBAD is associated with poor long-term outcomes including delayed false lumen expansion at 4 years in 20–50% of cases and a 30–50% cumulative 5-year mortality rate [6–8].
TEVAR can help stabilize TBAD in affected patients, promoting thrombosis of the false lumen and remodeling of the aorta [9, 10]. TEVAR is thus a common treatment for uncomplicated TBAD cases when seeking to reduce the long-term morbidity and mortality associated with this condition [11–16]. Despite this, there have been relatively few studies focused on comparing the relative efficacy of TEVAR and BMT in patients with acute uncomplicated TBAD, emphasizing the need for additional research on this topic.
Aim
This meta-analysis was developed with the goal of comparing TEVAR and BMT as primary treatments for patients with acute uncomplicated TBAD.
Material and methods
Study selection
This meta-analysis was conducted using the PRISMA guidelines [17], and has been registered at INPLASY.COM (No. INPLASY202380038).
Relevant studies published up to July of 2023 were identified by systematically searching the PubMed, Web of Science, and Wanfang databases with the following strategy: ((((endovascular repair) OR (TEVAR)) AND (medical)) AND (aortic dissection)) AND (type B).
Study inclusion criteria: (a) Types of studies: comparative analyses; (b) Diseases: acute uncomplicated TBAD patients; (c) Types of interventions: TEVAR vs. BMT; (d) Languages: no limitations.
Studies exclusion criteria: (a) single-arm studies; (b) non-human studies; (c) reviews, letters, and case reports.
Data extraction
Two authors independently extracted data from all eligible studies, resolving any inconsistencies via discussion with a third investigator. Data extracted from each study included baseline data (first author, year of publication, country, study design, patient numbers, age, gender ratio, comorbidities, extent of dissection, false lumen thrombosis rates, and follow-up intervals) and outcome data (duration of hospitalization, thrombosed/obliterated false lumen rates, re-intervention rates, early (≤ 30 day) mortality, late (> 30 day) mortality, aortic-related mortality, organ failure incidence, stroke incidence, rupture rates, retrograde type A dissection rates, and type I endoleak incidence).
Quality assessment
Randomized controlled trial (RCT) quality was evaluated with the Cochrane risk-of-bias tool, which was used to assign a low, high, or unclear risk of bias for each of the following categories: performance, attrition, detection, selection, reporting, and other bias.
The Newcastle-Ottawa Scale (NOS) [18] was employed to assess the quality of all other studies, with articles being scored based on criteria pertaining to selection (4 points), exposure (3 points), and comparability (2 points). High-quality studies were those with an NOS score ≥ 7.
Endpoints
The thrombosed/obliterated false lumen rate was the primary clinical endpoint for this study, while analyzed secondary endpoints included duration of hospitalization, re-intervention rates, early mortality, late mortality, aortic-related mortality, organ failure incidence, stroke incidence, rupture rates, retrograde type A dissection rates, and type I endoleak incidence.
Statistical analysis
RevMan v5.3 and Stata v12.0 were used to conduct these analyses. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for categorical variables, while continuous variables were compared using mean difference (MD) values and 95% CIs. Heterogeneity was assessed with the Q test and I2 statistic, with fixed-effect models being used unless significant heterogeneity was detected (I2 > 50%), in which case random-effect models were employed instead. A leave-one-out approach was used to identify sources of heterogeneity in sensitivity analyses. When assessing the potential for publication bias using funnel plots, this risk was considered low when all studies were within the narrow region of the funnel. When this was not the case, studies were evaluated with Egger’s test using p < 0.05 as a significance threshold.
Results
Study selection
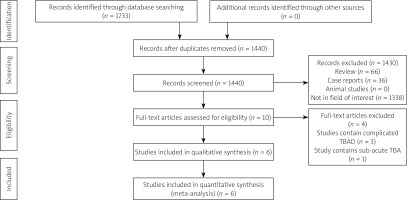
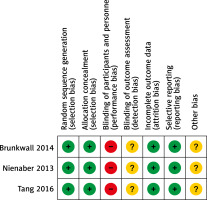
In total, the initial literature search identified 1,733 studies with potential relevance, of which 6 were included in the final meta-analysis [11–16], as detailed in Figure 1 (Table I). These studies included 3 retrospective analyses and 3 RCTs published between 2014 and 2023 in China, the USA, and Germany. A high risk of performance bias was noted for all 3 RCTs, together with an unclear risk of detection or other bias (Figure 2), whereas the NOS scores of the 3 retrospective studies were all 8.
Table I
Baseline data of included studies
| No. | First author | Year | Country | Design | NOS |
|---|---|---|---|---|---|
| 1 | Brunkwall [11] | 2014 | Germany | Randomized controlled trial | – |
| 2 | Lou [12] | 2023 | USA | Retrospective | 8 |
| 3 | Nienaber [13] | 2021 | Germany | Randomized controlled trial | – |
| 4 | Qin [14] | 2016 | China | Retrospective | 8 |
| 5 | Tang [15] | 2016 | China | Randomized controlled trial | – |
| 6 | Xiang [16] | 2021 | China | Retrospective | 8 |
These 6 studies enrolled 522 and 535 TBAD patients who underwent TEVAR and BMT treatment, respectively (Table II), with mean follow-up durations ranging from 12 to 72 months.
Table II
Baseline data of patients in the included studies
| Author | Groups | Patients (n) | Age [years] | Gender (M/F) | Comorbidities | Extent of dissection | False lumen thrombosis | Follow-up [months] | |
|---|---|---|---|---|---|---|---|---|---|
| Confined in thoracic aorta | Extended to abdominal aorta | ||||||||
| Brunkwall [11] | TEVAR | 30 | 63.6 | 21/9 | Not given | Not given | Not given | Not given | 12 |
| BMT | 31 | 63.3 | 27/4 | Not given | Not given | Not given | Not given | 12 | |
| Lou [12] | TEVAR | 50 | 54.4 | 26/24 | H, D, R, COPD | 12 | 38 | 19 | 51.6 |
| BMT | 96 | 58.6 | 72/24 | H, D, R, COPD | 36 | 60 | 25 | 51.6 | |
| Nienaber [13] | TEVAR | 72 | 60.3 | 62/8 | H, D, R, COPD | 8 | 64 | 26 | 69 |
| BMT | 68 | 60.1 | 56/10 | H, D, R, COPD | 5 | 63 | 23 | 69 | |
| Qin [14] | TEVAR | 184 | 58.8 | 161/23 | H, D, R, CAD | 16 | 168 | 72 | 72 |
| BMT | 154 | 62.9 | 141/13 | H, D, R, CAD | 18 | 136 | 57 | 72 | |
| Tang [15] | TEVAR | 41 | 52.0 | 22/19 | H, D, CAD | Not given | Not given | Not given | 12 |
| BMT | 41 | 52.2 | 20/21 | H, D, CAD | Not given | Not given | Not given | 12 | |
| Xiang [16] | TEVAR | 145 | 54.3 | 113/32 | H, D, R, CAD, COPD | 26 | 119 | 54 | 48 |
| BMT | 145 | 54.9 | 113/32 | H, D, R, CAD, COPD | 31 | 114 | 58 | 48 | |
Duration of hospitalization
Hospitalization duration was reported in 3 studies enrolling 379 and 395 patients who underwent TEVAR and BMT treatment, respectively [12, 14, 16]. Both groups exhibited a comparable pooled hospitalization duration (MD = 0.17; 95% CI: –2.24; 2.57, p = 0.89, Figure 3 A). Significant heterogeneity was detected (I2 = 96%), and the study conducted by Lou et al. [12] was established as the source thereof. When this study was omitted, the hospitalization duration remained comparable in both groups (p = 0.05).
Thrombosed/obliterated false lumen rates
Thrombosed/obliterated false lumen rates were reported in 3 studies enrolling 144 and 187 patients treated via TEVAR and BMT, respectively [12, 13, 15]. The TEVAR group exhibited a significantly higher pooled thrombosed/obliterated false lumen rate as compared to the BMT group (81.9% vs. 16.0%, p < 0.00001, Figure 3 B). Significantly heterogeneity was detected (I2 = 73%), and the study conducted by Lou et al. [12] was established as the source thereof. When this study was omitted, however, the pooled results remained unchanged (p < 0.00001).
Re-intervention rates
Re-intervention rates were reported in 4 studies enrolling 451 and 463 patients treated via TEVAR and BMT, respectively [12–14, 16]. In pooled analyses, both groups exhibited comparable re-intervention rates (4.9% vs. 7.1%, p = 0.12, Figure 3 C), and no significant heterogeneity was detected (I2 = 41%).
Early mortality
Early mortality (≤ 30 day) mortality was reported in 2 studies enrolling 329 and 299 patients treated via TEVAR and BMT, respectively [14, 16]. In pooled analyses, both groups exhibited comparable early mortality rates (0.6% vs. 2.3%, p = 0.09, Figure 3 D), and no significant heterogeneity was detected (I2 = 0%).
Late mortality
Late mortality (> 30 day) was reported in 3 studies enrolling 359 and 330 patients treated via TEVAR and BMT, respectively [11, 14, 16]. In pooled analyses, patients in the TEVAR group exhibited significantly lower rates of late mortality as compared to those in the BMT group (8.6% vs. 16.4%, p = 0.002, Figure 3 E), and no significant heterogeneity was detected (I2 = 0%).
Aortic-related mortality
Aortic-related mortality was reported in 3 studies enrolling 401 and 367 patients treated via TEVAR and BMT, respectively [13, 14, 16]. These pooled aortic-related mortality rates were significantly lower in the TEVAR group relative to the BMT group (5.5% vs. 15.3%, p < 0.0001, Figure 3 F), and no significant heterogeneity was detected (I2 = 0%).
Organ failure
Organ failure incidence was reported in 3 studies enrolling 379 and 395 patients treated via TEVAR and BMT, respectively [12, 14, 16]. Pooled organ failure rates were comparable in these two groups (0.5% vs. 1.5%, p = 0.36, Table III), and no significant heterogeneity was detected (I2 = 0%).
Table III
Meta-analytic pooled results for adverse events
Stroke
Stroke incidence was reported in 4 studies enrolling 451 and 463 patients treated via TEVAR and BMT, respectively [12–14, 16]. Pooled stroke rates were comparable in both groups (1.1% vs. 0%, p = 0.09, Table III), with no significant heterogeneity (I2 = 0%).
Rupture
Rupture rates were reported in 3 studies enrolling 370 and 340 patients who underwent TEVAR and BMT treatment, respectively [14–16]. Pooled rupture rates were significantly lower for patients who underwent TEVAR as compared to BMT (0% vs. 2.1%, p = 0.04, Table III), with no significant heterogeneity (I2 = 0%).
Retrograde type A dissection
Retrograde type A dissection rates were reported in 2 studies enrolling 329 and 299 TEVAR and BMT patients, respectively [14, 16]. These rates were comparable in both groups (1.8% vs. 3.0%, p = 0.95, Table III). Significant heterogeneity was detected (I2 = 61%), but sensitivity analyses could not be conducted as this endpoint only included 2 studies.
Type I endoleak
Type I endoleak incidence was reported in TEVAR patients in 3 studies [14–16], revealing a pooled incidence rate of 7% (Table III), with no significant heterogeneity (I2 = 0%).
Publication bias
Funnel plots were used to assess the risk of publication bias associated with key study endpoints. These plots revealed no significant risk of bias with respect to the thrombosed/obliterated false lumen rate, early mortality, late mortality, aortic-related mortality, organ failure incidence, stroke incidence, rupture rate, retrograde type A dissection rate, and type I endoleak rate endpoints. While Egger’s tests suggested that re-intervention rates were associated with a low publication bias risk (p = 0.9), this risk was deemed significant for the duration of hospitalization endpoint (p = 0.045).
Discussion
The present meta-analysis was developed as a means of comparing the safety and clinical efficacy of TEVAR and BMT as treatments for acute uncomplicated TBAD patients. Thrombosed/obliterated false lumen rates were the primary endpoint compared between these two treatment cohorts, as effective TBAD patient management hinges on the ability to prevent dissection rupture and to induce false lumen remodeling [19–21]. Patients who underwent TEVAR treatment exhibited significant improvements in pooled thrombosed/obliterated false lumen rates relative to patients who underwent BMT (81.9% vs. 16.0%). False lumen thrombosis is predictive of a reduction in TBAD patient event rates and favorable false lumen remodeling, and TEVAR treatment can aid efforts to seal the false lumen and to thereby promote thrombosis [22, 23].
TEVAR did not show significant superiority over BMT in this meta-analysis with respect to short-term patient outcomes. TEVAR did, however, significantly reduce late mortality and aortic-related mortality in treated patients, likely owing to the ability of this procedure to prevent rupture while promoting aortic remodeling. The survival benefits of TEVAR thus appear to require multiple years to manifest. Following TEVAR, the 1-year and 5-year survival rates of TBAD patients are reportedly in the range of 81.5–91.9% and 76–89.2%, respectively [16].
Stent graft scaffold insertion into the true lumen was associated with significantly increased true lumen diameter values, corresponding reductions in the diameter of the false lumen, and a 90% rate of complete false lumen thrombosis over a 5-year period in an analysis conducted by Nienaber et al. [13]. Brunkwall et al. [11] also assessed stent graft insertion-associated aortic remodeling, and observed that stented patients exhibited a significant increase in maximum true lumen size together with a smaller false lumen upon 1-year follow-up.
The present results suggest that TEVAR is not associated with a reduction in re-intervention rates, potentially because false lumen rupture is generally instantly fatal, so the opportunity for re-intervention is lost in these cases.
Comparable rates of other adverse events including stroke, organ failure, and retrograde type A dissection were noted when comparing TEVAR and BMT in this study, and all of these outcomes affected < 5% of patients. The TEVAR-specific incidence of type I endoleak occurred in 7% of cases in these pooled analyses, with the majority of cases being transient or relatively mild [16].
There are some limitations to this meta-analysis that should be considered. First, some of the included studies were not RCTs, potentially contributing to a higher risk of bias. In comparison to two prior meta-analyses comparing the TEVAR and BMT treatment of TBAD that included complicated TBAD [10] or sub-acute TBAD cases [1], however, the present analysis is subject to a somewhat lower risk of bias. Second, there was pronounced variation in follow-up periods among these studies, with intervals ranging from 12 to 72 months, contributing to potential bias pertaining to long-term outcomes. Third, certain endpoints exhibited a high degree of heterogeneity and the potential for publication bias. Appropriately constructed clinical studies will thus be essential to validate and expand upon the present findings.