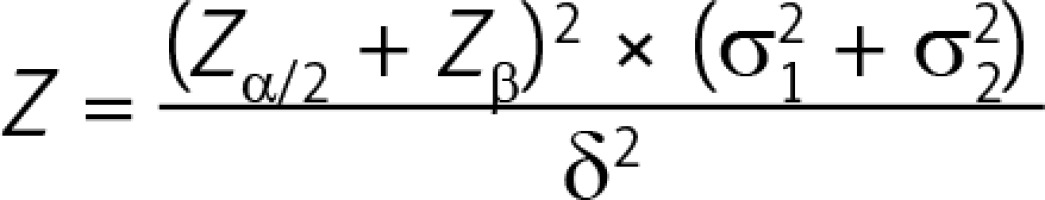Introduction
In 1997, Kehlet summarized contemporary methods for controlling postoperative dysfunction and indicated that multimodal interventions might help in reducing undesirable surgical outcomes and costs, as well as promoting recovery [1, 2]. That is now recognized as the very first description of the idea of enhanced recovery after surgery (ERAS). Based on it, ERAS was first adopted in colorectal cancer patients [3].
Despite its wide implementation in the fields of gastrointestinal surgery, hepatobiliary surgery, and cardiothoracic surgery [4], the application of ERAS in urology was late. While most studies focused on its effects on radical cystectomy [5–7], real-world trials on ERAS in renal cell carcinoma (RCC) were scarce.
Aim
The purpose of this study was to explore the feasibility and value of adopting ERAS in RCC patients treated with laparoscopic partial nephrectomy (LPN). Given that no consensus was reached on the ERAS protocol in the field of urology and variation of medical systems, the results might help deepen the understanding of ERAS in urosurgery and provide some concrete evidence for better policymaking.
Material and methods
Ethics statement and patient selection
This randomized controlled trial was designed and conducted according to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the authors’ institution (approval no. ZS-1559). The inclusion criteria were as follows: (I) patients with renal tumor who were about to receive laparoscopic partial nephrectomy as treatment; (II) 25–75 years old, regardless of gender; (III) no regional or distant metastasis; (IV) preoperative examinations showed no severe accompanying diseases or malnutrition. Exclusion criteria were: (I) those who did not meet the inclusion criteria; (II) other surgical methods or approach; (III) history of abdominal or retroperitoneal surgery; (IV) other situations assessed as factors interfering with the study. Data of postoperative length of stay (LOS), medical expenses, first-time water and food intake, return of anal exsufflation, postoperative nausea and vomiting (PONV), pain at different status and time, and postoperative complications were mainly analyzed.
Sample size
Recart et al. reported that the average LOS in their ERAS cohort was 41 ±11 h while that in the routine group was 59 ±11 h [8]. According to the bilateral test formula of two parallel groups:
where N is the number of required cases, Zα/2 is the Z value corresponding to α = 0.05 (Zα/2 = 1.96), Zβ is the Z value corresponding to the probability of type II error β (β = 0.1, Zβ = 1.28), δ is the difference of the means (δ = 18), and σ is the standard deviation in each group (σ1 = σ2 = 11). Here, we could calculate that the sample size in each group should be no less than 8 patients.
Finally, 60 patients were included after preliminary eligibility screening, and were randomly allocated to two cohorts: 31 in the ERAS optimization group and the other 29 in the traditional treatment group. All patients were treated by the same surgeon and had given their written informed consent before the operations.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Baseline characteristics and homogeneity
The R.E.N.A.L. score [9] in the traditional group was 7.28 ±1.62 (mean ± SD), while that in the ERAS group was 7.10 ±1.68. Student’s t-test showed that no difference in the tumor complexity was found between those two groups (t = 0.42, p = 0.677). Detailed baseline characteristics and homogeneity test results of the two groups can be seen in Table I. It could be interpreted that the demographic characteristics of the two groups, such as age, gender, and educational status, were comparable. Their preoperative clinical signs, laboratory tests, and physiological and biochemical indexes showed no difference as well. The conclusion could be drawn that the distribution of preoperative parameters in those two groups was balanced and comparable (p > 0.05).
Table I
Baseline characteristics and homogeneity test results of the two groups
| Category | Traditional group (mean ± SD) | ERAS group (mean ± SD) | χ2/t value/Z | P-value |
|---|---|---|---|---|
| Age [years] | 55.97 ±12.39 | 53.70 ±10.69 | 0.70 | 0.486 |
| Gender: | 0.01 | 0.919 | ||
| Male | 20 | 21 | ||
| Female | 9 | 10 | ||
| BMI | 25.24 ±3.28 | 26.63 ±3.84 | –1.494 | 0.141 |
| Body temperature [°C] | 36.37 ±0.38 | 36.13 ±0.35 | 0.411 | 0.683 |
| Heart rate [beats per minute] | 80.14 ±10.41 | 76.77 ±9.32 | 1.312 | 0.195 |
| Systolic pressure [mm Hg] | 132.52 ±13.93 | 137.70 ±15.86 | –1.332 | 0.188 |
| Diastolic pressure [mm Hg] | 80.24 ±7.71 | 78.70 ±11.05 | 0.621 | 0.537 |
| Respiratory rate [beats per minute] | 18.17 ±1.99 | 18.30 ±1.62 | –0.381 | 0.705 |
| Highest education level: | 0.191 | 0.849* | ||
| Illiterate: | 0 | 2 | ||
| Elementary school | 3 | 2 | ||
| Junior high school | 1 | 3 | ||
| Senior high school/equivalent | 14 | 10 | ||
| Bachelor’s degree | 5 | 7 | ||
| Postgraduate or above | 2 | 2 | ||
| Unknown | 4 | 5 | ||
| Previous medical history: | 0.098 | 0.754 | ||
| None | 12 | 14 | ||
| Mild illness | 17 | 17 | ||
| Leukocyte: | N.A. | 1.000** | ||
| Normal range | 28 | 30 | ||
| Abnormal range | 1 | 1 | ||
| Preoperative hemoglobin: | 0.308 | 0.579 | ||
| Normal range | 29 | 30 | ||
| Abnormal range | 0 | 1 | ||
| Preoperative creatinine: | N.A. | 1.000** | ||
| Normal range | 29 | 30 | ||
| Abnormal range | 0 | 1 | ||
| Total bilirubin: | 0.308 | 0.579 | ||
| Normal range | 26 | 29 | ||
| Abnormal range | 3 | 2 | ||
| Activated partial thromboplastin time: | N.A. | 1.000** | ||
| Normal range | 28 | 29 | ||
| Abnormal range | 1 | 2 | ||
| Prothrombin time: | 0.001 | 0.975 | ||
| Normal range | 27 | 30 | ||
| Abnormal range | 2 | 1 | ||
Perioperative management methods
Owing to the lack of consensus, the perioperative ERAS optimization protocol was drafted by summarizing published experience and then modified under real-world circumstances in our medical center.
Note that the routine protocol in the control group varied considerably from hospital to hospital; for instance, postoperative patients were strictly confined to bed for 1–2 weeks after surgery in some other studies [10, 11], while they were routinely required to start ambulation on postoperative day (POD) 2 in our hospital since years ago, to say nothing of the removal of the ureter catheter or drainage tube. This difference might explain our less favorable negative results in hospitalization expenses, postoperative LOS, and complications. Further exploration is presented in the Discussion section. Detailed perioperative management protocols of LPN patients are presented in Table II.
Table II
Perioperative management protocols for patients who have undergone laparoscopic partial nephrectomy
Quality control and statistical analysis
The software of EpiData version 3.1 (The EpiData Association) was used to input and manage the data. After the database was verified to be correct, a blind audit was conducted to determine the modification and confirmation of the statistical analysis plan, then the data were exported in the data format required by the statistical software. When data were output, a query table was generated for data range and logic check. Measurement data were described as mean ± standard deviation (mean ± SD), median, maximum, minimum, and quartile. Enumeration data were described as a percentage or constituent ratio. The independent-sample t-test was used to compare the measurement data with normal distribution and homogeneous variance; the independent-sample t-test was used to compare the measurement data with normal distribution but uneven variance; the Wilcoxon test (rank-sum test) was used to compare the measurement data with non-normal distribution or ordered enumeration data; the χ2 test in a contingency table was used to compare the non-ordered enumeration data. All statistical tests were performed in a two-sided test, and p-value < 0.05 was considered to be statistically significant. Statistical Product and Service Solutions version 24.0 (SPSS, IBM Corp.) was used to complete the statistical analysis.
Results
Clinical management and safety evaluation
All patients were treated by the same surgeon who was experienced in performing LRN. Student’s t-test indicated no statistically significant difference between the two groups in postoperative LOS, medical expenses, first-time drinking, return of anal exsufflation or fluid/semi-fluid food intake (p > 0.05). However, patients in the ERAS optimization group presented significantly earlier ambulation than those in the traditional treatment group (t = 2.743, p = 0.008). Detailed results are presented in Table III. Because most patients with renal cancers are older than 65 years old, we presented the subgroup analysis results of them in Table III as well (12 patients in the traditional group and 13 in the ERAS group). Note that patients over 65 presented a delayed return of normal function compared to their junior counterparts, while most data in the subgroup were comparable.
Table III
Results of different clinical management of the two groups
| Category | Traditional group(mean ± SD) | ERAS group (mean ± SD) | t value | P-value | Traditional group** | ERAS group** | t value** | P-value** |
|---|---|---|---|---|---|---|---|---|
| Postoperative length of stay [days] | 5.65 ±1.14 | 5.61 ±1.17 | 0.141 | 0.889 | 6.17 ±1.08 | 6.08 ±1.24 | 0.182 | 0.785 |
| Medical costs (converted to U.S. dollars*) | 3477.94 ±576.66 | 3581.73 ±672.09 | –0.64 | 0.525 | 3522.18 ±621.22 | 3710.12 ±581.26 | 0.117 | 0.657 |
| First-time drinking [h] | 28.52 ±17.26 | 28.15 ±17.10 | 0.175 | 0.862 | 29.75 ±11.33 | 29.15 ±13.20 | 0.225 | 0.982 |
| Recovery of anal exsufflation [h] | 35.36 ±17.36 | 34.43 ±21.26 | 0.187 | 0.852 | 37.42 ±13.36 | 38.15 ±16.94 | 0.219 | 0.789 |
| First-time ambulation [h] | 53.35 ±16.15 | 42.35 ±15.31 | 2.743 | 0.008 | 55.25 ±20.12 | 42.31 ±12.31 | 2.545 | 0.012 |
| Time of first intake of fluid food [h] | 41.17 ±16.39 | 38.57 ±18.19 | 0.581 | 0.563 | 42.17 ±18.17 | 39.92 ±16.26 | 0.651 | 0.612 |
| Time of first intake of semi-fluid food [h] | 53.14 ± 18.14 | 50.30 ±21.35 | 0.565 | 0.574 | 49.42 ±15.14 | 47.15 ±12.98 | 0.547 | 0.417 |
Outpatient clinic records showed that no patients reported incision bleeding, infection, poor healing, incontinence, thrombosis, cardiovascular events, or death 4 weeks after discharge.
Benefit evaluation
Results of pain degree at various status, nausea, vomiting at different time points were evaluated. The scoring system is presented in Table IV. It can be concluded the numeric rating scales (NRS) score of pain at rest and movement showed a tendency of changing with time (Figure 1); further analysis of variance of repeated measurements (R.M. ANOVA) indicated that the postoperative pain degree at rest within the first 12 h, postoperative pain at movement within the first 6 h and postoperative nausea within the first 4 h in the ERAS optimization group were significantly improved compared to those in the traditional treatment group (Table V). Although pain degree at in-bed turning over at the point of time of 12 h postoperatively in the ERAS group was significantly lower from that of the control group (t = 2.38, p = 0.018), the rest of the results did not show any significant difference.
Table IV
Scoring system in pain, nausea, and vomiting evaluation
Table V
Evaluations of pain at rest and ankle movement, nausea scores in both ERAS and traditional groups
Discussion
Renal cell carcinoma accounts for over 80% of all primary renal neoplasms, and over 400,000 people are diagnosed with RCC worldwide annually [12]. For patients with localized RCC, radical nephrectomy shows no superiority to partial nephrectomy in terms of oncologic outcomes [13–15]. On the other hand, enhanced recovery after surgery, developed from the idea of fast-track surgery, has been widely adopted in the field of general surgery [1–3]. ERAS is, in essence, a treatment protocol that calls for multidisciplinary and comprehensive interventions. The kernel of ERAS is a patient-oriented approach, adjusting perioperative management dynamically to alleviate both physical and mental trauma caused by operations, to lighten the burden of hospitalization economically [4]. With the renewal of this concept, relevant factors including anesthesia, nursing, and social workers have also been added [16, 17].
Although the application of ERAS in patients undergoing radical cystectomy has been individually reported, its prospects in renal cancer, prostate cancer, and urinary calculi are still unexplored [18]. In our view, radical cystectomy, especially with intestine-related urinary reconstructions, would often bring greater surgical impacts and thereafter raise more interest from a range of disciplines, while treatment such as LPN is more urologist-dominant and less cross-disciplinary. However, one cannot conclude that ERAS in the field of urology is less important just because there is little research on it.
In our experience, preoperative education is the key to ERAS. Thorough preoperative education could help patients understand the treatment process better, reduce their psychological pressure and improve their compliance. In different groups, we adopted different preoperative education strategies; namely, education was given to patients in the traditional treatment group orally in a guidance-compliance model, while a cooperative education model was established using multimedia platforms in the ERAS optimization group. Although we did not design a specific scale for evaluating preoperative education, it was obvious that patients in the ERAS group showed a better understanding of the postoperative rehabilitation process and a higher degree of compliance than those in the traditional treatment group; for instance, patients in the ERAS group showed more care about ambulation and nutrition than their counterparts. Note that there was no difference in educational background.
Traditionally, preoperative long-term fasting was regarded as protection in avoiding emesis and mis-inhalation during anesthesia, however, more and more supportive studies showed that preoperative shorter-term fasting and oral carbohydrate liquid intake would not increase the incidence of intraoperative mis-inhalation [4, 16]. Moreover, it could help reduce perioperative fluid load and stabilize blood glucose. In this study, patients in the ERAS group with normal bowel function would not receive mechanic bowel preparation as the operation was performed in the retroperitoneal space. Analysis showed that there was no significant difference in the recovery of anal exsufflation. We assumed that this was because when anesthesia, surgical time, and other elements were similar, the only variable, polyethylene glycol solution, was mild and its effect on the intestinal tract was not significant. Hence, the conclusion that bowel preparation could be modified to be less intensive in general cases might be drawn, and therefore clinicians could improve patients’ satisfaction and safety.
Previous studies had not discussed the role of analgesia in ERAS of LPN. Our study found that patients with the optimized analgesia protocol, namely prophylactic analgesia and short-acting opioids, reported less feeling of pain, both at rest and movement, and consequently an average of 11-hour earlier ambulation. Patient-controlled analgesia (PCA) is a kind of pain management technique in which anesthesiologists set the pain management program in advance according to the degree of pain and physical condition of patients, by which the patients could adjust and control the timing and dosage of receiving the painkiller according to their own needs. According to the different approaches of administration, PCA can be mainly divided into patient-controlled intravenous analgesia (PCIA) and patient-controlled epidural analgesia (PCEA). In this study, the technique of PCIA was adopted for postoperative analgesia because of the following reasons: (1) The block plane of PCEA in renal surgery should be around the 6th thoracic vertebral level; the high thoracic epidural block might have higher risks of hypotension and increase the difficulty of perioperative management; (2) PCEA should have higher possibility of urinary retention, which might affect the results of early urinary catheter removal; (3) PCIA would be easier for nursing; (4) cultural reasons. Pain and ambulation were regarded as two mutually reinforcing factors. For one thing, the decrease of pain would be conducive to faster ambulation. For another, earlier walking might help distract patients’ attention, promote intestinal peristalsis, prevent the occurrence of intestinal adhesions, and thereafter, lower the incidence of intestinal spasm. At the same time, earlier walking would contribute to the psychological recovery of patients.
The occurrence of postoperative nausea was reported to be up to 80% and vomiting was 30%, regarding surgery and anesthesia techniques, as well as predisposing patient status [19]. Postoperative nausea and vomiting (PONV) was sometimes omitted because of its mild symptoms and self-limited clinical features, yet it could be lethal in some extreme cases [20]. In this study, prophylactic anti-nausea drugs were provided to patients on a routine basis rather than the traditional demanding-prescription mode. The results indicated that the early incidence (postoperative 0–6 h) of nausea in the ERAS group was significantly lower, while the late incidence of PONV in the two groups showed no significant difference, which was consistent with previous studies [10]. We considered that these different results were partly because of the nature of retroperitoneal surgery; namely, the vasovagal reflex might be calmed in the first 6 h after surgery by anti-nausea drugs, while later with the return of patients’ water intake and upcoming PONV pathophysiological climax (24 h postoperatively), cases of PONV occurred more often and intensively [19, 20]. For this reason, we might assume that our ERAS protocol was more effective in control of early nausea while more measures were needed for longer-term palliation in PONV.
Unlike other studies, this study revealed no difference in the costs and LOS between the two groups. We were initially surprised because the ERAS group should theoretically recover better and faster than the traditional group and therefore should have shorter postoperative LOS and lower hospital costs [4, 7]. Through an in-depth literature study, we found that the medical expenses in our medical center were much lower than those extracted from other studies in the same country, either in the traditional group or the ERAS group [10, 11]. Moreover, the LOS of both groups in our medical center was similar to that in the reported ERAS cohorts. Reasons for these situations might be that the control group in most ERAS studies was “idealized and traditionalized”. For example, in some study designs [10, 11], patients with LPN in the control group were required to stay in bed for 1–2 weeks before they could walk for the first time, while in real-world circumstances this is less likely to happen. For those patients, their hospitalization days and expenses would inevitably be different. Our view is that surgeons have more or less acquired the idea of ERAS in clinical practice and the management has been updated accordingly; hence, statistical difference on LOS and expenses might be less possible to be found. Besides, because the medical system varies from region to region, the LOS would differ as well. For instance, patients from the developed regions would have better access to the outpatient clinic for removal of their drainage tube or to the community hospital for further treatment. Consequently, instead of paying attention to positive results derived from old and abandoned settings, we suggest the importance of shortening the LOS and costs in ERAS studies should be less emphasized and a conclusion should be made based on the real-world status quo.
Despite its strengths, our study is not lacking in limitations. That is, its sample size limits the power to detect a differential result of the two groups. Moreover, the protocol of ERAS is not customized to match the characteristics of nephrectomy; therefore, some variables and parameters might be omitted. Finally, bias might occur during the transformation of some non-quantitative and subjective variables to quantitative values using scales.
Conclusions
ERAS has imperceptibly influenced surgeons’ strategy making in the past 20 years. This real-world trial showed that the adoption of ERAS in laparoscopic partial nephrectomy could contribute to less pain at rest and movement, earlier postoperative walking, less early-stage nausea, and better satisfaction. Length of stay and expenses might not be the most important observation indices in large medical centers because of the variations of the medical system and clinical situations. However, larger-sample clinical trials are welcomed from all levels of hospitals, and a customized ERAS protocol in the field of urology is needed.