Introduction
Carotenoids are a subfamily of isoprenoid pigments that represent diverse colors such as red, yellow, and orange (Saini and Keum, 2019). The color of carotenoids depends on the number of conjugated double bonds in their structure (Novoveská et al., 2019). Carotenoids are classified into two main groups according to the presence or absence of oxygen in their molecules: xanthophylls or oxygen-containing carotenoids (astaxanthin, canthaxanthin, cryptoxanthin, flavoxanthin, lutein, neoxanthin, violaxanthin, and zeaxanthin) and carotenes or unoxygenated (oxygen-free) carotenoids, i.e., pure hydrocarbons with no oxygen (α-carotene, β-carotene, γ-carotene, δ-carotene, ε-carotene, and lycopene) (Langi et al., 2018). Carotenoids are also classified based on the number of carbons in their structure, namely C30, C40, C45, and C50 carotenoids (Yabuzaki, 2017).
Carotenoids have diverse physiological functions. For example, they are precursors of the phytohormone abscisic acid in plants (Sathasivam et al., 2020). Carotenoids are also one of the most important components of the photosynthesis apparatus in light harvesting (400–550 nm) (Novoveská et al., 2019; Ram et al., 2020). Additionally, carotenoids play a vital role in the cellular photoand UV-protection by dissipation of excess energy, especially in photosynthetic and radiation-resistant organisms (Gabani and Singh, 2013; Tian et al., 2019). These stresses lead to the formation of reactive oxygen species (ROS) in cells that can be neutralized by carotenoids (Ozougwu, 2016; Langi et al., 2018; Novoveská et al., 2019). In addition, there are numerous health-related beneficial effects of carotenoids on humans and animals (Nabi et al., 2020). Carotenoids have antioxidant, anti-inflammatory, anticancer, antiaging, antibacterial, and antiviral activity. They are also precursors of vitamin A. Carotenoids also enhance the immune system and protect against cardiovascular, cataract, and neurodegenerative diseases (Gyawali and Ibrahim, 2014; Saini and Keum, 2019). In addition to the pharmaceutical industry, the global market of carotenoids had good growth potential in the last several years because of wide applications of carotenoids in food, animal feed, beverages, diet supplements, and cosmetic industries (Molnár et al., 2006; Bogacz-Radomska et al., 2020).
Although carotenoids provide many advantages, animals and humans cannot synthesize these pigments and must uptake them exclusively from the diet (Rodriguez-Concepcion et al., 2018). Carotenoids are often (80–90%) synthesized chemically because of their high purity and cost-effectiveness (Ram et al., 2020). Importantly, the chemical reactions of carotenoid synthesis often include some nonbiological byproducts that could have adverse influence on the environment and human health; therefore, natural carotenoids are receiving more attention (Hamidi et al., 2017). More than 1100 carotenoids are produced by a wide array of organisms, including bacteria, archaea, yeast, fungi, microalgae, and plants (Ram et al., 2020). Most of the natural carotenoids are extracted from plants such as annatto, carrot, grapes, paprika, and tomato (Zhao et al., 2019). However, plant pigments have some limitations such as seasonal production, poor water solubility, and instability (Venil et al., 2013). Because of these properties, more consideration has been given to microbial pigments as the production of microbial pigments is more cost-effective and is not affected by climatic conditions (Malik et al., 2012). Moreover, bacteria can produce C30, C40, C45, and C50 carotenoids; however, eukaryotes can produce only C40 carotenoids (Yabuzaki, 2017). Previous studies have shown that various microorganisms, including microalgae and cyanobacteria (Haematococcus pluvialis, Microcystis aeruginosa, Spirulina, Anabaena variabilis, Oscillatoria limosa, and Nostoc commune), yeasts (Rhodotorula sp., Rhodosporidium sp., Sporobolomyces sp., Phaffia rhodozyma, Haematococcus pluvialis, and Xanthophyllomyces sp.), and bacteria (Streptomyces chrestomyceticus, Flavobacterium sp., Paracoccus carotinifaciens, Dietzia natronolimnaea, and Gordonia jacobeae), produce carotenoids efficiently (Novoveská et al., 2019; Zhao et al., 2019; Ram et al., 2020).
In the present study, we investigated the production of carotenoid by a radiation-resistant microbial strain, phylogenetically close to Dietzia maris, isolated from oil-contaminated soils. The genus Dietzia includes gram-positive short rods or coccobacilli, catalase-positive, oxidase-positive, and nonacid-fast bacteria belonging to Actinomycetales and is closely related to the genus Rhodococcus (Tao et al., 2007). The colonies of all the strains of the genus Dietzia have been reported to be pigmented ranging from yellow to coral-red, and are small, circular, and convex (Venugopalan et al., 2013). The strains of Dietzia are the most promising sources of C40 carotenoids, especially an orange-red ketocarotenoid named canthaxanthin (CTX, C40H52O2, 4,4′-diketo-β-carotene). Dietzia maris NIT-D and Dietzia natrono-limnaea HS-1 have been reported as the best microbial strains for CTX production (Khodaiyan et al., 2007; Venugopalan et al., 2013; Gharibzahedi et al., 2014a). CTX is approved for feed application in the United States, Canada, and the European Union. It has antioxidative, anticancer, antidermatosis, and anti-inflammatory properties (Gharibzahedi et al., 2014a; Bogacz-Radomska et al., 2020).
Previous studies have shown that the optimization of the formulation of fermentation medium and culture conditions have a significant influence on the production of carotenoids by microbial strains (Zhang et al., 2018; Mapelli-Brahm et al., 2020). The optimization of the production of microbial metabolites must be performed for each strain separately due to their metabolic and physiological differences (El-Banna et al., 2012). Moreover, the need to reduce the production cost has led to the use of low-cost industrial byproducts such as cheese whey as a fermentation medium in the synthesis of value-added microbial metabolites (Roukas et al., 2015). Cheese whey is a byproduct of the dairy industry, and approximately 60–90 g of 100 g milk is converted to whey during cheese production (Irkin, 2019). Therefore, cheese whey represents a significant source of milk nutrients (nearly 55%), including lactose (66–77%, w/w), proteins (8–15% w/w), and minerals salts (7–15% w/w) (Charalampia et al., 2019). Unfortunately, cheese whey is known as the most important pollutant of the dairy industry due to the high organic load (mainly high content of lactose and proteins) (Lappa et al., 2019).
In this context, the present study aimed to investigate the effect of various sources of carbon and nitrogen as well as different concentrations of whey medium on carotenoid production by a new radiation-resistant strain of Dietzia maris. The antioxidant, cytotoxic, and antibacterial activities of the studied pigment were also evaluated.
Materials and methods
Isolation of the radiation-resistant strain
Tryptone-glucose-yeast extract (TGY) medium (pH = 7.2) containing tryptone (5 g/l), glucose (1 g/l), and yeast extract (5 g/l) was used to isolate the radiationresistant strain from petroleum-contaminated soil surface at the depth of 1–5 cm (Khuzestan, Iran). For isolating UV-resistant microbial strains, 1 g of soil samples was added to 50 ml TGY broth medium and incubated at 30EC for 3 days. After the incubation period, 100 μl of the broth medium was cultured onto TGY agar medium by using the spread plate technique. The components of TGY agar medium (pH = 7.2) were tryptone (5 g/l), glucose (1 g/l), yeast extract (5 g/l), and agar (15 g/l). The plates were then exposed to 10 J/cm2 of UV radiation at 254 nm for 30 min from a distance of 14 cm to a UV lamp (UV CROSSLINKER® (CL-508)). After radiation treatment, the plates were placed in an aluminum foil and incubated at 30EC for 3–10 days. Subsequently, the second UV exposure was performed using 15 J/cm2 of UV radiation for 45 min. The microbial strains that grew after the second treatment were regarded as UV-resistant strains. Among the isolated strains, the most resistant one against UV radiation that produced the pigmented colony similar to the colony before the UV exposure was selected for further analysis (Gholami et al., 2015).
Identification of the isolated strain
Morphological and biochemical characteristics of the isolated strain were determined according to the standard methods (Gilardi 1967; Xue et al. 2008). The molecular identification of the strain was performed using the amplification of the 16S ribosomal DNA (rDNA) using the universal primer 27F (5′–AGAGTTTGATYMTGGCTCA–3′) and 1492R (5′–CGGTTACCTTGTTACGACTT–3′). The boiling method was used to extract bacterial genomic DNA from a pure culture (Junior et al., 2016). The PCR reaction mixture (25 μl) comprised 12.5 μl PCR Master Mix (2×, Cinna Gen), 0.5 μl forward primer (10 pmol), 0.5 μl reverse primer (10 pmol), 1 μl extracted DNA, and 10.5 μl deionized water. The PCR cycling conditions were as follows: initial denaturation at 94°C for 5 min, 30 cycles of denaturation at 94°C for 45 s, annealing at 55°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 5 min. The PCR product was then electrophoresed on a 1% agarose gel (Etemadifar et al., 2016). The sequence of the amplified rDNA fragment was determined by Sanger sequencing (Korean Macrogen Company) and then compared with sequences from other bacterial species previously reported in the GenBank database (www.ncbi.nlm.nih.gov/Blast.cgi, NCBI). Multiple alignments of bacterial 16S rDNA sequences and the phylogenetic analysis were conducted using the Muscle program and the maximum likelihood method using the MEGA 6 software package, respectively.
Optimization of pigment production
The amount of carotenoids synthesized in the presence of 1 g/l glucose, sucrose, maltose, starch, xylose, sorbitol, and raffinose was evaluated in a broth medium containing yeast extract (1 g/l) and dipotassium phosphate (KH2PO4, 0.1 g/l). The amount of carotenoids synthesized in the presence of 1 g/l yeast extract, tryptone, peptone, casein, ammonium sulfate, calcium nitrate, potassium nitrate, and ammonium nitrate was evaluated in a broth medium containing 1 g/l glucose and 0.1 g/l KH2PO4.
The effect of different concentrations of whey medium (Pegah Company, Iran), i.e., 4, 5, and 6% w/v, on the pigment production of the isolated strain was also determined. The whey medium was sterilized in three steps: first, autoclaving at 110°C for 15 min; second, filtering (Whatman No. 40 filter paper) and centrifuging at 4000 rpm for 20 min, and third, autoclaving at 110°C for 15 min.
The inoculum was prepared using a loopful of pure culture in a 5 ml TGY broth medium (pH 7.2) until it reached an optical density (OD) equal to that of a 0.5 McFarland standard (1–2 × 108 CFU/ml). It was then inoculated into the abovementioned media at the concentration of 1% v/v and incubated at 30°C for 5 days (120 rpm).
The produced biomasses were extracted, and the total carotenoid content was evaluated for each fermentation medium following the procedures described below.
Extraction of carotenoid pigment
For each fermentation medium, a 10 ml sample was taken and centrifuged at 4000 rpm for 10 min at 4°C to remove the supernatant. The cell pellets were washed twice with saline (0.9% w/v NaCl in deionized water) and centrifuged again at 4000 rpm for 10 min at 4°C. Subsequently, the cell pellets were resuspended in 2 ml of pure methanol by vortexing for 10 min and incubated at 10°C for 45 min. The mixture was then centrifuged at 4000 rpm for 10 min to obtain the supernatant. The resulted supernatant was collected, and the residual biomass was remixed with the solvent. The addition of solvent to the bacterial pellet and the subsequent steps were repeated until the pellet became completely colorless. Finally, the resulting supernatants were dried at 40°C and stored at 4°C for further analysis (Zamanian and Etemadifar, 2017). In addition to methanol, other solvents including acetone, ethanol, chloroform, and N-hexane, were also separately used to extract the carotenoid pigment from the fermentation media.
Measurement of biomass and total carotenoid content
The amount of the produced carotenoid in the 10 ml fermentation medium was evaluated according to equation:
In equation, A474, Vs, and represent the total carotenoid maximum absorbance in the solvent, the sample solution volume, and the total carotenoid specific absorption coefficient in a 1 cm cell for a 1% solution, respectively (Nasri Nasrabadi and Razavi, 2010).
To determine the amount of the produced microbial biomass, 100 ml of each fermentation medium was centrifuged at 4000 rpm for 5 min. The pellet was then dried at 70°C for 72 h and weighed. For the whey medium, the microbial biomass was calculated by subtracting the weight of the microbial pellet from the weight of the pellet from an uninoculated whey medium.
Antioxidant activity of the pigment using ferric reducing antioxidant power (FRAP)
To determine the antioxidant activity of the pigment extract using the ferric reducing antioxidant power (FRAP) assay, various concentrations of the pigment (0.5–10 μg/ml) were mixed with 1 ml ddH20, 2.5 ml sodium phosphate buffer (200 mM, pH 6.6), and 2.5 ml potassium ferricyanide (1% w/v). The mixture was then incubated at 50°C for 20 min, following which 2.5 ml trichloroacetic acid (10% w/v) was added and the solution was mixed. The final mixture was centrifuged at 2000 rpm for 10 min. Subsequently, 1 ml ferric chloride (0.1% w/v) was mixed with a 10 ml sample mixture containing 5 ml supernatant and 5 ml sterile ddH20. The absorbance of the mixture was immediately measured at 700 nm. Ascorbic acid was used as a positive control, and all the analyses were performed in triplicate. The half-maximal effective concentration (EC50), i.e., the amount of pigment that decreased the absorbance of ferricyanide by 50%, was determined using a dose-response linear regression plot (inhibition percentage on the vertical axis against pigment concentration on the horizontal axis) (Benzie and Szeto, 1999).
Antioxidant activity of the pigment using DPPH
To determine the antioxidant activity of the pigment extract using 2,2-diphenyl-1-picrylhydrazyl (DPPH), different concentrations of the pigment (0.05–10 mg/ml) were mixed with 0.1 mM DPPH methanolic solution (1 ml). The mixture was then incubated in a dark room for 30 min at room temperature. Subsequently, the absorbance of the mixture was measured at 517 nm. Ascorbic acid was used as a positive control and all the analyses were performed in triplicate. The half-maximal effective concentration (EC50), i.e., the amount of pigment that decreased the absorbance of DPPH by 50%, was determined using a dose-response linear regression plot (inhibition percentage on the vertical axis against pigment concentration on the horizontal axis) (Sharma and Bhat, 2009)
Cytotoxic activity of the pigment
The cytotoxic activity of the extracted pigment was measured using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. In this test, the viability of cells was analyzed based on the ability of their mitochondrial NAD(P)H-dependent oxidoreductases to reduce MTT to its purple insoluble form (formazan) in the presence of the pigment. In the present study, the MTT assay was performed using the human fibroblast (HBF) cell line (IBRC C10459, Iranian Biological Resource Center) based on the protocol described previously (Rezaeeyan et al., 2017).
Antimicrobial activity of the pigment
The agar well diffusion method was used to evaluate the antibacterial activity of D. maris pigment extract against Staphylococcus aureus and Escherichia coli in Muller-Hinton agar (MHA) plates. For this purpose, 50 μl of carotenoid extract, 100 μl of bacterial suspension (equivalent to 0.5 MacFarland standard), and 50 μl methanol as the control were used. All the analyses were performed in triplicate, and the mean of the diameter of inhibition growth was reported as the antibacterial activity of the pigment extract after 24 h incubation at 37°C.
Results
Microbial strain identification and phylogenetic analysis
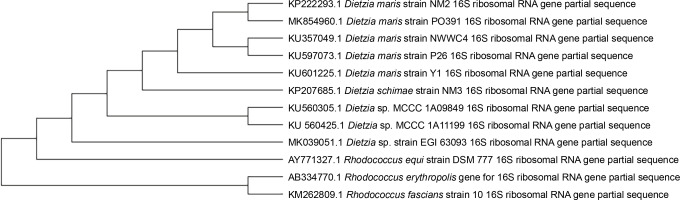
After the second stage of the UV tolerance screening method, 10 UV-resistant microbial strains were isolated from an oil-contaminated soil, and one of these strains was selected for further analysis based on the highest number of grown colonies (1.2 × 103 CFU/ml). The studied strain was gram-positive, nonmotile, catalase-positive, coccoid bacterium with orange pigmented colonies on TGY agar medium. The partial 16S rRNA gene of the isolated strain was sequenced and deposited in GenBank under the accession number KP222293. The strain showed 99.2% similarity to Dietzia maris based on the results of BLAST analysis. The phylogenetic dependencies also showed that the strain had a close relationship with D. maris (Fig. 1).
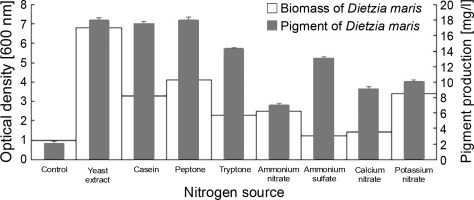
Effect of different nitrogen sources on biomass and pigment production
All nitrogen sources had a significant influence on the pigment production of the studied strain as compared to the control, which had no nitrogen sources (Fig. 2). The nitrogen sources increased the pigment production of D. maris in the following order: yeast extract (18 mg/l) = peptone (18 mg/l) > casein (17.5 mg/l) > tryptone (14.3 mg/l) > ammonium sulfate (13 mg/l) > potassium nitrate (10 mg/l) > calcium nitrate (9 mg/l) > ammonium nitrate (7 mg/l). The nitrogen sources also increased the microbial biomass content of D. maris in the following order: yeast extract (6.8 g/l) > peptone (4.1 g/l)> potassium nitrate (3.4 g/l) > casein (3.3 g/l) > ammonium nitrate (2.5 g/l) > tryptone (2.3 g/l) > calcium nitrate (1.4 g/l) > ammonium sulfate (1.2 g/l). The results of the effect of different nitrogen sources on the biomass and carotenoid production of D. maris are shown in Table 1.
Table 1
Dietzia maris biomass and pigment production in the presence of various fermentation media formulation
Fig. 2
Effect of different nitrogen sources on the biomass and carotenoid production of Dietzia maris
Yeast extract, peptone, and casein increased the pigment production of D. maris by approximately 9-fold. These nitrogen sources also increased the microbial biomass by approximately 6.8-, 4.1-, and 3.3-fold, respectively. Therefore, it can be concluded that the carotenoid pigment content had a direct relationship with the bacterial biomass in the presence of organic nitrogen sources. However, this relationship was not observed for the inorganic nitrogen sources in the fermentation medium.
The results showed that potassium nitrate (10 mg/l), calcium nitrate (9.5 mg/l), and ammonium nitrate (7 mg/l) had the lowest effect on pigment production, although potassium nitrate increased the microbial biomass by approximately 3.4-fold. Therefore, nitrate salts were not considered as effective nitrogen sources in the pigment production of the studied strain.
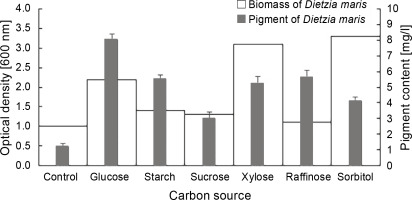
Effect of different carbon sources on biomass and pigment production
The results of the effect of different carbon sources on the biomass and carotenoid production of D. maris are shown in Table 1. All carbon sources had a significant influence on the pigment production of the studied strain as compared to the control, which had no carbon sources (Fig. 3). The maximum pigment production increased by approximately 6.6-fold in the presence of glucose (P = 0.01). The carbon sources increased the pigment production of D. maris in the following order: glucose (8 mg/l) > raffinose (5.6 mg/l) = starch (5.5 mg/l) = xylose (5.2 mg/l) > sorbitol (4.1 mg/l) > sucrose (3 mg/l). However, only glucose (2.2 g/l), xylose (3.1 g/l), and sorbitol (3.3 g/l) increased the microbial biomass at a significance level when compared with the control (1.1 g/l). The biomass production in the medium containing sucrose (1.3 g/l) or raffinose (1.1 g/l) was not significantly different from that of the control (1 g/l). Therefore, in contrast to the organic nitrogen sources, the pigment production did not have a direct relationship with the bacterial biomass in the presence of the studied carbon sources. For example, the highest increase in biomass was obtained in the medium containing sorbitol, but it had the lowest positive effect on pigment synthesis (3 mg/l).
Effect of different concentrations of whey on biomass and pigment production
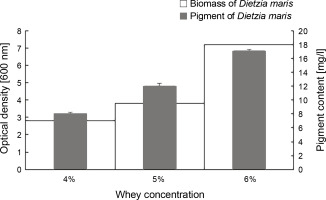
The results of the effect of different whey concentrations on the biomass and carotenoid production of D. maris are shown in Table 1. The whey medium at the concentration of 6% w/v increased the microbial biomass (7.2 g/l) and carotenoid production (17 mg/l) of D. maris more than that observed for the other studied concentrations of whey (Fig. 4).
Evaluation of antioxidant, antibacterial, and cytotoxic properties of pigment
The results of D. maris antioxidant activity using FRAP and DPPH tests are shown in Table 2. The antioxidant capacity of D. maris carotenoid extract was evaluated as EC50 3.30 mg/ml and EC50 28.46 μg/ml in the DPPH and FRAP assays, respectively. At the concentration of 10 mg/ml, the pigment extract decreased the absorbance of DPPH by approximately 91%, which was higher than that of the control, i.e., ascorbic acid (89%) in the DPPH method. The results also revealed that a dose-dependent effect of D. maris carotenoid pigment in both the FRAP and DPPH assays, i.e, the antioxidant activity of D. maris carotenoid pigment increased with the increase in the pigment concentration.
Table 2
Dietzia maris antioxidant activity
| Concentration [mg/ml] | Pigment DPPH assay (inhibition percent) | Concentration [μg/ml] | Pigment FRAP assay (OD700 nm) |
|---|---|---|---|
| 0.05 | 18 | 0.5 | 0.035 |
| 0.1 | 26 | 2 | 0.07 |
| 0.5 | 31 | 4 | 0.13 |
| 1 | 43 | 6 | 0.14 |
| 5 | 68 | 8 | 0.17 |
| 10 | 91 | 10 | 0.19 |
D. maris carotenoid pigment had no cytotoxic effect against the HFB cell line. The percent of viable cells in the presence of 50, 100, 200, and 500 mg/ml of D. maris carotenoid pigment was 93, 88, 82, and 77%, respectively.
The results of the agar well diffusion method to evaluate the antibacterial activity of D. maris pigment extract against S. aureus and E. coli showed that the extract has no antibacterial effects on the tested bacterial strains on the basis of absence of inhibition zones around S. aureus and E. coli colonies.
Discussion
Carotenoids are extensively used in various industries, including pharmaceuticals, food, animal feed, beverages, diet supplements, and cosmetics (Cheng et al., 2020). Their high commercial demand has led to the supply of these compounds through chemical synthesis technology (Ram et al., 2020). However, the adverse effects of synthetic carotenoids, such as carcinogenicity, toxicity, and teratogenicity properties, have resulted in increasing attention being directed toward natural carotenoid sources, including microorganisms (Galanakis, 2016). Previous studies have shown that higher carotenoid pigment production in the microbial strains could be related to a higher ability to resist UV radiation (Mohana et al., 2013; Sajjad et al., 2017). Therefore, the carotenoid pigment production by a radiation-resistant strain of the genus Dietzia was investigated in the present study. The genus Dietzia has broad potential biotechnological applications such as production of pigments, biosurfactants, bioemulsifiers, and extracellular polymeric substances (EPSs) as well as in bioremediation of pollutants, hydrocarbons, and crude oil (Click and Van Kampen, 2009; Liu et al., 2009; Nakano et al., 2011; Gharibzahedi et al., 2014a). However, there are few studies on the production of carotenoid pigment in this genus (Khodaiyan et al., 2007; Tao et al., 2007; Nasri Nasrabadi and Razavi, 2010; Gharibzahedi et al., 2013; Venugopalan et al., 2013; Zamanian and Etemadifar, 2017). Various microbial species or strains need different carbon and nitrogen sources for their growth and pigment production. Therefore, the optimum fermentation media and culture conditions for pigment production must be determined for each strain separately (Zhao et al., 2019). Hence, in the present study, the optimization of carotenoid production of a newly isolated UV-resistant bacterial strain was conducted in the presence of various carbon and nitrogen sources.
The results showed that all the studied carbon and nitrogen sources had significant effects on the biosynthesis of pigment production in D. maris when compared with the control. The maximum amount of biomass and carotenoid pigment produced by D. maris was obtained in the fermentation medium containing 1 g/l glucose and 1 g/l yeast extract (18 mg/l). Similar results were obtained in previous studies. Khodaiyan et al. (2007) reported that the most effective carbon source for growth and CTX production by D. natronolimnaea HS-1 was glucose (Khodaiyan et al., 2007). Gharibzahedi et al. (2012) found that glucose and mannose had a positive influence on CTX biosynthesis and the growth of a mutant of D. natronolimnaea HS-1 (Gharibzahedi et al., 2012). In contrast, the growth of D. maris NIT-D was inhibited by glucose at concentrations higher than 15 g/l (Goswami et al., 2012). The carotenoid production of Neurospora intermedia, Xanthophyllomyces dendrorhous, and Kukoria marina was decreased in the presence of glucose (Mitra et al., 2015; Alcaíno et al., 2016; Gmoser et al., 2018). However, this phenomenon was not observed in our study, and the pigment production increased by 6.6-fold in the fermentation medium containing glucose as the carbon source.
Among inorganic nitrogen sources, ammonium sulfate and potassium nitrate showed better effects on the pigment production of the studied strain. The effectiveness of ammonium sulfate and potassium nitrate in the production of carotenoids was also reported previously for other microbial strains (El-Banna et al., 2012; Mohana et al., 2013). In this study, despite an increase in the microbial biomass in the presence of some inorganic nitrogen sources, the organic nitrogen sources, especially yeast extract, peptone, and casein, had more positive effects on the pigment production of D. maris. Similarly, Khodaiyan et al. (2008) and Gharibzahedi et al. (2014b) found that yeast extract had a significant effect on the production of CTX by D. natronolimnaea HS-1 (Khodaiyan et al., 2008; Gharibzahedi et al., 2014b). Overall, D. maris produced more carotenoid pigment under the optimized condition when compared with the maximum amount of carotenoid pigment produced by Rhodotorula glutinis (1.9 mg/l) and D. natronolimnaea HS-1 (14.67 mg/l) (Khodaiyan et al., 2007; El-Banna et al., 2012).
Industrial wastes such as whey, cane molasses, potato starch, and corn steep liquor are the cost-effective nutrient sources for the production of carotenoids or other microbial metabolites (Kot et al., 2019; Galal and Ahmed, 2020; Zheng et al., 2021). Among them, whey contains lactose and casein that serve as carbon and nitrogen sources for pigment production (Khodaiyan et al., 2008; Ribeiro et al., 2017; Charalampia et al., 2019). Herein, the studied strain showed good biomass and carotenoid production in the presence of lactose, casein, and whey medium (maximum 17 mg/l). Because the amount of carotenoid production of D. maris in the whey medium was not very less than that in the optimized fermentation medium, the whey medium can be considered as an economical fermentation medium for the production of carotenoids using D. maris. Cheese whey is considered as a promising fermentation medium for carotenoid production; however, only a few studies have addressed this issue to date (Ribeiro et al., 2017; Lappa et al., 2019; Bonett et al., 2020). Dietzia natronolimnaea HS-1 (2.87 mg/l) and Rhodotorula mucilaginosa (11.3 mg/l) are some of the microorganisms that produced carotenoid through fermentation in cheese whey (Khodaiyan et al., 2008; Marova et al., 2017). Compared to these strains, D. maris in the present study produced more carotenoid pigments using cheese whey (17 mg/l).
EC50 represents the carotenoid extract concentration that decreases the initial DPPH concentration by 50%; therefore, the lower the EC50 value, the higher is the antioxidant activity (Ron et al., 2018). The results of the antioxidant activity of D. maris carotenoid extract revealed EC50 3.30 mg/ml in the DPPH assay and EC50 28.46 of the DPPH test reported in previous studies for Pseudomonas argentinensis (31.13 mg/ml), Serratia rubidaea (10.31 mg/ml), Arthrobacter sp. G20 (4.5 mg/ml), Dietzia schimae (1.58 mg/ml), Rhodothermus marinus DSM 4252T (223.1 μg/ml), and Spirulina platensis (66.6 to 204.5 μg/ml), the antioxidant activity of the strain in the present study was moderate (Pawar et al., 2015; Afra et al., 2017; Zamanian and Etemadifar, 2017; Ron et al., 2018). All the concentrations of the carotenoid extract in the present study showed DPPH scavenging or ferric reducing power activities, and the antioxidant activity increased with an increase in carotenoid concentration.
In conclusion, D. maris is a promising industrial strain for the production of carotenoid pigment because this strain produced a significant amount of carotenoid. In addition, the carotenoid extract of D. maris showed antioxidant activity; moreover, the pigment was nontoxic and noninhibitory to the human fibroblast cell line and normal flora. Further studies should be conducted to improve the biosynthesis of carotenoid production of D. maris.