Introduction
Pneumonectomy, primarily performed for non-small cell lung cancer (NSCLC) resection, occasionally extends to lung metastases or infectious indications [1–4]. However, a consequential and rare complication, pleural empyema following pneumonectomy (PPE), carries an incidence ranging from 2% to 16% and a mortality rate that can soar up to 50% [1]. Effective prognosis and management of PPE pivot on the presence of bronchus stump insufficiency (BSI), commonly necessitating a comprehensive approach involving thoracotomy for fistula closure, pleural debridement, lavage, drainage, and reinforcement of the compromised bronchial stump with viable tissue flaps [1, 5–8]. Alternative minimal invasive techniques, such as occluder implantation, have surfaced as viable interventions for select cases [9]. In instances devoid of BSI, a diverse array of treatments exists, ranging from straightforward pneumonectomy cavity drainage and lavage to more invasive procedures such as thoracotomy and open thoracostomy. Recent years have witnessed the emergence of a minimally invasive technique – video-assisted thoracic surgery (VATS) – for managing PPE cases with minimal or absent BSI, demonstrating promising outcomes [10, 11].
Aim
This study aims to synthesize and elucidate the documented experiences drawn from existing literature concerning the utilization of VATS in the treatment of PPE, aiming to highlight its efficacy and role within the clinical landscape.
Material and methods
Formation of the study population
The study population was assembled by meticulously examining documented cases within the existing literature pertaining to the subject under investigation.
Search strategy of published studies
A comprehensive search was conducted on Medline (via PubMed) covering the period from 1995 to 2020 to identify relevant studies germane to the theme of interest. Search terms encompassed a combination of subject headings and keywords such as “pneumonectomy”, “bronchus stump insufficiency”, “bronchopleural fistula”, “VATS”, “video-assisted thoracic surgery”, “pleural empyema”, and “postpneumonectomy”. Additional relevant reports were identified through manual scrutiny of bibliographies within initially extracted articles. Eligibility of retrieved references and assessment of potential biases in each study were independently appraised by two authors (Dr. Grapatsas and Dr. Ehle). Data collection from each report was conducted independently by the two aforementioned authors. In instances of discordance, resolution was sought through arbitration by a third review author (Dr. Leivaditis). Subsequently, full texts of the remaining retrieved articles were independently scrutinized by the authors to ascertain the presence of pertinent information. Inclusion of the final retrieved articles was subject to unanimous approval by all authors. The literature review prioritized identifying the frequency of procedures per patient, bacterial isolation within the thoracic cavity, postoperative mortality rates, and the necessity for thoracostomy.
Results
Study selection process
A systematic search of Medline was conducted, initially yielding a pool of potentially relevant studies. Subsequent screening of titles or abstracts led to the identification of six studies that met all predefined inclusion criteria and were thus included for detailed analysis in this study (Table I).
Table I
Overview of the existing literature on video-assisted thoracic surgery for treatment of postpneumonectomy pleural empyema
Characteristics of the study population
Upon comprehensive review of existing literature, a cohort of 63 patients was identified, with a broad age range spanning from 16 to 74 years. The frequency of thoracoscopic interventions per patient ranged from 1 to 7 across the included studies. Bacterial presence within the intrathoracic cavity was documented in five studies, with prevalent pathogens predominantly affiliated with the Staphylococcus and Streptococcus genera. The duration of hospitalization varied considerably, from 9 to 94 days, among the study population. Notably, the observed mortality rate within this cohort was 1.5%. Thoracostomy was necessary in three documented cases, signifying the need for additional intervention in managing postpneumonectomy pleural empyema.
Other key findings
The identified studies provided additional insights into postoperative complications, including the development of bronchus stump insufficiency, the necessity for further surgical interventions, such as open revisions, and the utilization of various strategies for managing persistent empyema. The collective data extracted from the analyzed studies shed light on the multifaceted nature of postpneumonectomy pleural empyema, encompassing diverse patient demographics, clinical presentations, and management strategies. These findings underscore the complexity of managing this condition and highlight the importance of tailored approaches in achieving optimal patient outcomes.
Discussion
Postpneumonectomy pleural empyema
PPE is a perilous and intricate thoracic surgical complication, carrying a staggering perioperative mortality ranging from 25% to 75%, often contingent upon the presence of BSI. Occurrences of isolated PPE devoid of BSI are exceptionally rare, manifesting variable periods of diagnosis that can span from a mere week to an astonishing 35 years after pneumonectomy [1, 10, 12–16].
Therapeutic strategies for prevention of PPE
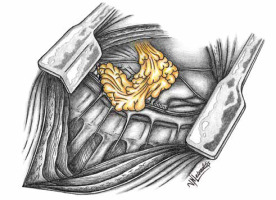
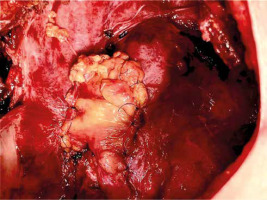
Numerous therapeutic modalities have been expounded in the literature [12–16] for managing isolated PPE, aligning with the patient’s general condition at admission or diagnosis [1]. Diverse measures encompassing the augmentation of the bronchial stump, creation of Clagett thoracostomy, reduction of the postpneumonectomy space with pedicled muscle or omentum, and, infrequently, thoracoplasty have been recommended [1, 10, 11, 17, 18]. Covering the bronchus stump with vital tissue holds promise. Figures 1 and 2 schematically depict the post-pneumonectomy coverage of the bronchus stump with a vital pericardial fat flap. Figure 3 further presents a case wherein the bronchial stump is entirely covered by such a flap.
Figure 1
Schematic representation illustrating the mobilization of a pericardial fat flap while preserving its blood supply
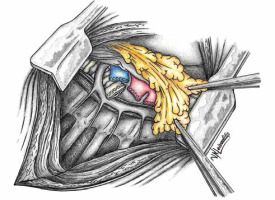
Outcomes and recurrence rates
Absence of BSI often leads many authors to hesitate in pursuing aggressive surgical treatment for isolated PPE. Despite therapeutic interventions, managing isolated PPE poses challenges, often resulting in high recurrence rates and elevated perioperative mortality [10, 11, 17, 18]. For instance, Pairolero et al. reported a 56% success rate with intrathoracic insertion of pedicled muscle flaps. However, this intervention led to a mortality rate of 18.75% (n = 3 patients) and a recurrence of PPE in 25% of cases (n = 16) [19]. Similarly, Weber et al. treated PPE using thoracostomy, without recurrence but with a mortality rate of 29% [20].
Alternative therapeutic approaches
Some authors advocate employing a chest drain and pleural cavity lavage as an alternative management strategy for isolated PPE. Although successful in several cases, this method has been associated with prolonged hospital stays. However, it is crucial to acknowledge that intrapleural lavage may not consistently achieve comprehensive sterilization of the pleural cavity. Persistent microbial growth within the chambered postpneumonectomy cavity despite lavage attempts can lead to recurrent PPE [12–16].
Role of video-assisted thoracic surgery
In recent years, VATS has evolved as a pivotal tool in treating pleural empyema. VATS exhibits advantages over intrapleural lavage, as elucidated by Wait et al. in a randomized study in 1997. VATS showed reduced empyema recurrences, shorter chest tube durations, and decreased hospital stays for patients [21]. VATS enables the removal of newly formed fibrin and membranes from the operated hemithorax, preserves chest wall continuity, and allows direct vision lavage of the pleural cavity.
Despite its advantages, the role of VATS as a definitive standard treatment for isolated PPE remains somewhat ambiguous in the literature [21]. This uncertainty might be attributed to the limited number of pertinent publications, with a mere six identified in our review (Table I) [10, 12–16]. Notably, among these studies, the largest patient cohort comprised only 18 individuals [10, 15]. Early contributions to this discourse, such as the work by Dr. M. Ernst and Dr. Ch. Nies in 1999, showed promising outcomes through multiple thoracoscopic lavages in two PPE patients without BSI, yielding favorable bacteriological and cosmetic results [13]. Similarly, Hollaus et al. reported successful VATS treatments in 9 patients, albeit raising concerns about the complete debridement of the pneumonectomy cavity, particularly in areas such as the costodiaphragmatic recess. However, we argue that such technical challenges identified in 1999 may have been overcome with the accumulated experience in VATS techniques [12]. Additionally, Gossot et al. highlighted that complete debridement of the pneumonectomy cavity might not invariably be essential in PPE [14]. Notably, Bagan et al. and Ng et al. advocate for VATS as the primary treatment for isolated PPE, recommending postoperative thoracic cavity lavage as prophylaxis. Both studies reported favorable outcomes, with only one death and the requirement of thoracostomy in a total of 23 patients [15, 16].
Nevertheless, in complex cases involving persistent infection or suspected PPE recurrence despite intrapleural lavage, further aggressive therapeutic interventions rather than additional lavage should be considered. Such scenarios might necessitate re-thoracotomy, employing a vital tissue flap to cover the bronchial stump, and intrathoracic vacuum-assisted closure (VAC) therapy to manage persistent intrathoracic infections [1].
The outcomes observed with VATS for isolated PPE show promising trends in both morbidity and mortality rates. Across studies involving persistent PPE, a thoracostomy was only required in three cases collectively [14, 15]. Looking forward, the future of PPE management could benefit from larger-scale, comprehensive studies elucidating VATS as the definitive treatment for isolated PPE, addressing technical intricacies, and further exploring the role of advanced adjunctive therapies to optimize patient outcomes. This could pave the way for refining guidelines and establishing VATS as the gold standard in the management of isolated PPE. However, the wide spectrum of PPE presentations, from acute septic shock to late chronic phases, poses complexities that extend beyond the scope of this discussion. While minimally invasive techniques such as VATS hold promise, their application must be judiciously considered within the context of individual patient characteristics and disease severity. While definitive guidelines for VATS in PPE remain elusive due to the condition’s variability, recognizing its potential within the treatment armamentarium underscores the need for continued research and refinement in this area.
Limitations: The study has several limitations worth noting. Primarily, the retrospective nature of the investigation introduces inherent limitations in terms of data collection, accuracy, and potential bias. Secondly, the study is encumbered by the small sample size across individual studies, limiting the generalizability and statistical robustness of the findings. Additionally, a substantial limitation lies in the considerable heterogeneity observed within the patient population. The inclusion of patients spanning distinct time periods, undergoing procedures across diverse surgical departments and countries, contributes to a considerable variability that may impact the study’s outcomes and conclusions. Moreover, the possibility of selection bias across all studies is a concern. Notably, there seems to be a tendency to select stable patients for VATS procedures, while those with urgent surgical needs due to sepsis are more inclined towards thoracotomy. Consequently, the outcomes derived from our study should be regarded solely as observational in nature, warranting cautious interpretation and highlighting the need for further comprehensive investigations.
Conclusions
VATS is emerging as a viable and effective minimally invasive therapeutic approach for managing PPE in the absence of BSI. This technique not only preserves the integrity of the chest wall but also demonstrates favorable outcomes with limited mortality and morbidity. However, in instances where PPE persists despite VATS intervention, the consideration of surgical revision via thoracotomy becomes imperative. The success of VATS in addressing PPE without BSI underscores its potential as a primary therapeutic modality, supporting its inclusion in the armamentarium of approaches for managing this challenging postoperative complication.