Introduction
Ovarian cancer is the most lethal gynaecologic cancer worldwide [1], and the median age at diagnosis is around 63 years in most developed countries. It is more common in older than younger women [1, 2]. The incidence and death rates due to ovarian cancer are higher in developed than in developing countries. Nevertheless, the incidence rates and related deaths in Western Europe and Northern America have either declined or plateaued since the beginning of the 21st century [2]. Incongruously, ovarian cancer incidence and the related mortality rate have increased in industrial and some developing countries, especially those that have witnessed economic transition [1]. This could be due to westernized lifestyle, a significant decrease in the number of pregnancies, decreased family size and the reliance on milk formula to feed new babies instead of breastfeeding [3].
The majority of ovarian cancers arise from the epithelium cells of the ovary [4]. Thus, based on the appearance of the epithelium, these neoplasms are divided into five major carcinoma histotypes [4]: high-grade serous carcinoma which represents around 68% of the cases, clear cell carcinoma (12%), endometrioid carcinoma (11%), mucinous carcinoma (3%), and low-grade serous carcinoma (3%). In addition, 3–4% of epithelial ovarian cancer cases are due to common alleles identified by genome-wide association studies [5, 6]. Ovarian cancer is an asymptomatic disease; thus despite the development in screening technology, surgical procedures and chemotherapy, most ovarian cancer cases are only identified at advanced stages [3]. This is because there is no single, reliable, specific screening procedure for detecting ovarian cancer at an earlier stage [1, 3].
Advanced stages of ovarian cancer (III, IV) are associated with poor prognosis and a significant decrease in survival rate compared with those diagnosed at stage I [7], although survival rate may vary according to the different disease histotypes [8]. Furthermore, women who are younger than the average age for ovarian cancer at diagnosis (younger than 63 years) have better survival rates than those diagnosed at an older age than the average. Risk factors for ovarian cancer can be divided into modifiable and nonmodifiable factors (Table 1). Nonmodifiable risk factors include older age [9], genetics [6, 10], family history [11, 12], history of previous cancers [13] and late menopause [14]. Modifiable risk factors include nulliparity [15], hormone replacement therapy (HRT) [16], tobacco smoking [17, 18] and dietary fat [19, 20]. Controversial factors include obesity [21, 22], talc powder exposure [23–25], radiation exposure [26, 27], infertility [28–30] and fertility medications [28, 31, 32]. This review provides a detailed description of the aetiology, epidemiology and risk factors of ovarian cancer.
Table 1
Well-established risk factors for ovarian cancer
| Authors and year | Risk factor | Reported risk |
|---|---|---|
| Bandera, et al., 2016 [9] | Older age | As age increases women’s chances of having ovarian cancers increase. Regardless of their race/ethnicity, more than two thirds of ovarian cancer cases are diagnosed after the age of 50 years |
| Kuchenbaecker, et al., 2017 [10] | BRCA 1 BRCA 2 | The cumulative risk of ovarian cancer to age 80 years was 44% (95% CI: 36–53) for BRCA1 and 17% (95% CI: 11–25) for BRCA2 carriers |
| Lee, et al., 2016 [34] | HRT | Current HRT users have an increased risk for serous and endometrioid ovarian carcinoma particularly of long duration (10 years or longer) |
| Gaitskell, et al., 2018 [35] | Nulliparity | Nulliparous women have a 24% higher risk of ovarian cancer compared with women who had one child |
| Faber, et al., 2013 [18] | Tobacco smoking | Current cigarette smokers have 31% increased risk of invasive mucinous [OR = 1.31 (95 % CI: 1.03–1.65)] and 83% for borderline mucinous ovarian tumours [OR = 1.83 (95 % CI: 1.39–2.41)]. The risk of borderline serous ovarian tumours was 30% higher among former smokers [OR = 1.30 (95 % CI: 1.12–1.50)] |
| Shanmughapriya, et al., 2016 [19] | Dietary fat | Women who consumed dietary fat have more than six-fold increase in ovarian cancer risk [OR = 6.286 (95% CI: 0.779–50.701)] compared with women with a vegetarian diet |
Material and methods
The current article has reviewed English literature for epidemiological, pathological and genetics related studies on ovarian cancer. The main aim of the present study is to explore, update and expand on the current risk factors and epidemiology of ovarian cancer [33]. Following the definition of the main aim of the current study, we have used a set of keywords including ovarian cancer, aetiology of ovarian cancer, epidemiology, prevalence, incidence, morbidity, mortality and risk factor in the search for related articles. In order to evaluate risk factors for ovarian cancer, we looked at every factor separately and thoroughly in order to define factors with the strongest relationship, controversial factors and those that are weakly related. Additionally, duties such as searching for related articles, and collecting and summarizing the main outcomes were divided between authors equally. We first conducted a broad search in order to collect all available articles on ovarian cancer from 1970 to the end of 2022. In order to do so, we performed a deep internet search, using PubMed, Google Scholar, Scopus and Web of Science databases. Later, we narrowed our search and included only full-text, English articles on ovarian cancer between 1990 and 2022. We considered well-designed clinical and epidemiological studies including prospective and retrospective cohorts, case control studies and other observational studies on ovarian cancer. Furthermore, we included high quality reviews, meta-analyses and systemic reviews. However, case reports, clinical and epidemiological studies with methodological errors and/or those with weak study design were not considered. Studies with vague and/or conflicting conclusions were also excluded. Opinions on this review’s topic have been discussed deeply and shared with other experts and colleagues in the field of gynaecology oncology.
The aetiology of ovarian cancer
Ovarian cancer is a cancer of postmenopausal women, and it is rare in women below the age of 40 years. Thus, it is classically described as a disease of older women. The median age for women with ovarian cancer ranges from 60 to 65 years in most developed coun- tries [2]. As life expectancy has increased in most countries worldwide, and because the incidence rate of ovarian cancer increases with age, more and more postmenopausal women will have ovarian cancer [3]. Approximately 90% of ovarian cancers develop from ovarian surface epithelial cells [36]. The aetiology and precursor lesions of epithelial cancers are multifactorial, partially because epithelial cancers tend to have a complex and heterogeneous histology that defies a simple biological explanation [37]. Nevertheless, about 10–15% of ovarian cancer is due to genetics, including mutations in BRCA genes, hereditary nonpolyposis colon cancer or Lynch syndrome and Peutz-Jeghers syndrome [38]. Furthermore, the initiation of the disease is influenced by specific reproductive variables as well as familial or personal characteristics [35]. Ovarian cancer is a complex disease because it can develop at all ages and from different cell types in the ovary, including oocytes, granulose cells, theca interstitial cells, and the surface of epithelium [35]. Thus, the classification of ovarian cancer is difficult, even if attention is confined to epithelial cancer.
The latest WHO classification lists five major histotypes of ovarian cancer: high-grade serous carcinoma, low-grade serous carcinoma, mucinous carcinoma, endometrioid carcinoma and clear cell carcinoma. Because these histotypes arise from different cells of origin, cell lineage-specific diagnostic immunohistochemical markers and histotype-specific oncogenic alterations are used to confirm the morphological diagnosis [39]. Compared with tumours of other organs, ovarian neoplasms are composed of heterogeneous histologic types; not only epithelial tumours but also sex-cord stromal tumours and germ cell tumours develop. While the exact mechanism of ovarian cancer is still not yet elucidated, several theories have been proposed to describe disease development and aggression, as seen in Figure 1 [3].
Fig. 1
The most accepted hypotheses explaining the development of ovarian cancer and their supporting evidence [3]
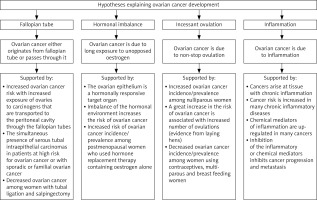
Ovarian cancer is a multifaceted disease that involves many factors. Nevertheless, it is currently accepted that, even when the main risk factors exist in an individual, the existence of an environmental factor is crucial to trigger the initiation of the disease [40].
The epidemiology of ovarian cancer
The incidence rate of the disease is lower than in other gynaecologic cancers; however, it is the most fatal of all gynaecologic cancers and accounts for more than two thirds of all deaths due to gynaecologic cancers, mainly because most cases are diagnosed at later stages [41]. Worldwide, the prevalence of ovarian cancer varies markedly, being highest in Western Europe and Northern America, intermediate in Southern and Eastern Europe and South America and lowest in the Middle East and Asia. Generally, the differences between countries of highest and lowest prevalence of ovarian cancer can be explained by racial, reproductive, socioeconomic and cultural differences [3, 35, 42].
The higher prevalence of ovarian cancer in developed compared with developing countries can be due to many factors. The first is the significant increase in life expectancy in developed countries. The second is the significant decrease in fertility rate (decreased family size) in comparison with some developing countries where large families still exist and women still have the motivation for a higher numbers of pregnancies [43]. Further, women of developed countries are less prone to practise breastfeeding [3], which was found to be protective against ovarian cancer and its protective effect can last for almost 30 years after stopping [43]. Thirdly, in developed countries, westernized lifestyle has a major impact on increasing most types of cancers including ovarian cancer. In addition, increased daily intake of fatty diet and dense caloric food, which is common in developed countries, is also connected to most types of cancers. The increased prevalence of being overweight and obesity in developed countries has a significant correlation with cancer incidence. In women with ovarian cancer, obesity is mostly related to a decrease in quality of life and the five-year survival rate [20].
In developed countries, ovarian cancer incidence varies across different racial and ethnic groups. African American women are 40% less prone to develop ovarian cancer compared to White American women [9]. Part of these variations is due to the existence or the absence of BRCA1 and BRCA2 mutations [6, 10, 44, 45], which is more prevalent in Ashkenazi Jews [46]. Generally, BRCA1 mutations were significantly more common in White (2.9%) than Black (1.4%) cases and in Jewish (10.2%) vs. non-Jewish (2.0%) cases [47]. In contrast, BRCA2 mutations were slightly more prevalent in Black women (2.6%) than their White counterparts (2.1%) and were more frequent in non-Jewish (2.3%) compared to Jewish women (1.1%) [47]. Factors such as number of children, cigarette smoking and dietary fat may also contribute to the racial differences. Furthermore, the results can be influenced by the methodology or type of epidemiological studies. While prospective cohort studies may under-evaluate the relationship between dietary fat and ovarian cancer, over-evaluation of this relationship is one of the major drawbacks in retrospective cohort and case control studies due to recall bias. Indeed, recall bias is always expected when risk factors (i.e. diet type) are assessed after cancer has been diagnosed [41].
In the USA, death rates due to ovarian cancer represent 4% of the total death rate due to women’s cancers, whereas death due to breast cancer accounts for 15% of the total death due to women’s cancers [48]. The differences between races may expand to include significant differences in age at diagnosis, stage at diagnosis and survival rate. Black women are usually diagnosed at a later stage and their five-year survival rate is less (40.7% vs. 44.1%) than in White women [49]. African American women had the worst survival rate compared to all other ethnic groups in the USA. Compared to White American women, African Americans have a 56% higher mortality rate [HR = 1.56 (95% CI: 1.01–2.39)], and Hispanics 41% higher [HR = 1.41 (0.98–2.04)], while Asian women have a survival rate that is 11% higher than that of White women [HR = 0.89 (0.61–1.31)] [9].
The incidence and prevalence of ovarian cancer have been distorted worldwide in different ways. Thus, in developed countries, the prevalence of ovarian cancer is distorted by an increase in certain types of surgery. These include hysterectomy [50, 51], tubal ligation [51, 52], opportunistic salpingectomy [53], oophorectomy [54], and unilateral and bilateral salpingo- oophorectomy [51, 55]. Furthermore, differences in exposure to preventive factors have a great impact on the incidence/prevalence of ovarian cancer. In developed and many developing countries, millions of women are using contraceptives to avoid falling pregnant. Contraceptive use is associated with a significant reduction of ovarian cancer [42, 56]. In contrast, in many developing countries, as societies are going through a transition towards a more sedentary lifestyle, the risk of ovarian cancer is rising. Parents are satisfied with a smaller family size and a smaller number of children, whereas women are also no longer practising breastfeeding, and ovarian cancer incidence has increased [3]. In Africa, especially southern countries of Africa such as South Africa, Botswana and Swaziland, the prevalence of ovarian cancer has been affected by an increase in the prevalence of fatal diseases such as human immune deficiency virus (HIV/AIDS) and tuberculosis. This is because women with these diseases usually die before reaching the median age of ovarian cancer [57].
Established risk factors
Established risk factors are closely associated with disease incidence rate; thus it has classically been used to identify and to predict individuals and families at risk [58]. Because ovarian cancer is a multifaceted disease, the identification of an individual at high risk is based mainly on medical background, with family history being the most important risk factor. Risk factors for ovarian cancer include older age, genetics, family history, history of other cancers, nulliparity, late menopause, HRT, tobacco smoking and dietary fat.
Older age
Ovarian cancer is a cancer of old women; therefore the majority of women with ovarian cancer (≈75%) are diagnosed after menopause [9]. Older age at diagnosis is associated with poor prognosis and a significant decrease in survival rate [41]. The median age ranges between 60 and 65 years in most countries [59]. This range may however be confounded by ethnicity of targeted participants (i.e. BRCA1/2 carriers may develop the disease earlier), sample size (i.e. small vs. large sample size) and the way participants were selected and grouped (i.e. private vs. general hospital). Thus, some studies have suggested that the median age of ovarian cancer (represented by disease peak) may emerge earlier. Lower age at diagnosis is probably related to the hereditary status of breast and ovarian cancer [60]. Thus, being previously diagnosed with breast cancer is associated with significantly lower age at diagnosis of ovarian cancer [61], and the opposite is also true [62]. Other factors that affect the median age at diagnosis may include the way of recruiting patients and whether they were recruited from general or private hospitals. Medical status of the volunteers also affects the median age, as individuals with ovarian and/or breast cancer and those with a family history of cancer are most willing to join studies on cancer, which creates an assortment bias. The race of participants may have an impact on the median age for ovarian cancer. African Americans have a lower median age than the international median [63].
Genetics
Ovarian cancer is part of a phenotype of two distinct familial cancer syndromes. These are hereditary breast/ovarian syndrome and Lynch syndrome (or non-polyposis colorectal cancer). These mutations are associated with higher risk of ovarian cancer, representing 10% of epithelial ovarian cancer causes and tend to promote ovarian cancer development at a younger age [6, 10, 44, 45]. Although breast cancer mutations BRCA1 and BRCA2 are mostly observed among Ashkenazy Jews [64], previous studies have detected these mutations in different ethnicities as well [47, 65]. Compared with a lifetime risk of 2% for the general population, the average cumulative risks by age 70 for ovarian cancer among BRCA1 or BRCA2 mutations is 59% (95% CI: 43–76) and 16.5% (95% CI: 7.5–34) respectively [6], in line with what was reported later. Corresponding breast cancer lifetime risks were 72% for BRCA1 and 69% for BRCA2 carriers [10]. These results have provided solid evidence that the risk of cancer in women with BRCA1/2 mutations increases with an increasing number of affected first and/or second-degree relatives, suggesting that genetic or other familial related factors modify cancer risks for BRCA1/2 mutation carriers [66]. In patients diagnosed with ovarian cancer due to BRCA gene mutations, 84% of patients were reported to be BRCA1 carriers while the rest (16%) were BRCA2 carriers [67].
Through experience and the results of the ongoing prospective cohort study (data not published), our data have shown differences in the incidence, aggressiveness and invasion rates between BRCA1 and BRCA2. Although their functions are closely related as they cooperate together and share duties when repairing DNA damage and commencing their effect at a single time point (same age), BRCA1 and BRCA2 are totally different. Most importantly, BRCA1 gene mutation is associated with a significantly higher risk of ovarian cancer (two- to three-fold) than BRCA2. Consequently, when analysing cases classified by age group and type of gene mutations (BRCA1 vs. BRCA2), the relative risk (RR) of ovarian cancer varies significantly across all age groups (Fig. 2), in line with results reported previously [6]. Furthermore, the RR among patients with BRCA1 increased sharply, reaching its peak around menopause, while in BRCA2 gene carriers it reaches its lowest effect around this point (Fig. 2). Not surprisingly, triple negative patients (oestrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2–) are mostly associated with the BRCA1 mutation, in accordance with what was reported previously for women with breast and ovarian cancer [66].
Fig. 2
BRCA1 mutation carriers display a higher risk towards the development of ovarian cancer across all age groups
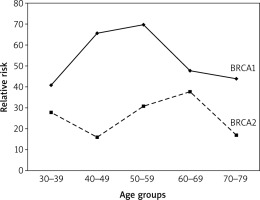
A recent study reported that the loss of the BRCA1 gene led to transcriptional reprogramming in tumour cells and cell-intrinsic inflammation involving type I interferon (IFN) and stimulator of IFN genes (STING). BRCA1-mutated tumours are thus T-cell inflamed at baseline [68]. This could explain partially the aggressiveness of cancer among BRCA1 mutation carriers compared to those with BRCA2 mutation. A previous study [60] showed that the median age at diagnosis is significantly younger for BRCA1 than BRCA2 patients (54 vs. 62 years respectively). Compared with the general population, carriers of nonpolyposis colorectal cancer mutation have a 13% lifetime risk of ovarian cancer, which is far less than the risk for colorectal (68%) and endometrial (62%) cancers [69].
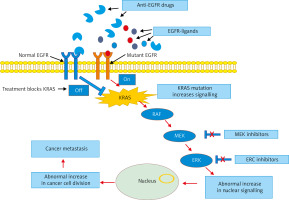
When it comes to the treatment of ovarian cancer, genetic mutations such as KRAS mutation represent the greatest challenge compared with other mutations (i.e. BRCA1/2). This is because KRAS is associated with serious alterations in the normal cell metabolism and clinical resistance to chemotherapy. KRAS is an affecter molecule responsible for signal transduction from ligand-bound epidermal growth factor receptor (EGFR) to the nucleus, and thus it plays a crucial role in many cancers, including ovarian cancer (Fig. 3). Previous studies have shown that high intake of vitamin C is associated with a significant decrease in cells harbouring KRAS or BRAF mutations [70]. In a normal healthy cell, EGFR facilitates normal growth and differentiation; thus cells can divide and grow normally (Fig. 3). However, when a deleterious mutation exists (i.e. cancer), the number of mutant receptors increases and cells continue to grow and divide abnormally. At the cellular level, EGFR plays a key role in cancer development and metastasis, promoting tumorigenesis and survival [71].
Fig. 3
Pathway analysis demonstrating drug resistance in patients with KRAS mutation. KRAS mutation contributes to the resistance through constitutive activation of epidermal growth factor receptor (EGFR) downstream signalling cascades regardless of EGFR blockade. In normal EGFR, the use of anti-EGFR drugs blocks KRAS and stops cancer cells’ division and metastasis, while in mutant EGFR, anti-EGFR drugs are not effective
Family history
Compared to families with no history of ovarian cancer, previous studies have reported a three- to four-fold higher rate of ovarian cancer among first-degree relatives diagnosed with the disease [11, 12]. This risk is, however, a lifetime risk; the familial risk may decline with the age at which the relative was affected and with the age of the woman at risk. Familial clustering of ovarian cancer with other cancers is most often attributable to a shared genetic basis of cancer and environmental factors [40, 62]. Mutations in BRCA1/2, which confer a significant risk for ovarian cancer, also predispose individuals to breast, prostate and other cancers [72]. A previous study showed that the RR of ovarian cancer among sisters of women diagnosed with ovarian cancer before the age of 55 years was 5.2 in comparison with 3.6 for sisters of women diagnosed after the age of 55 years [73].
History of previous cancer
Women with a history of other cancers are at an increased risk of developing ovarian cancer [13, 27]. Compared with women of the general population, breast cancer survivors have a 24% increased risk of developing ovarian cancer [13]. The risk of ovarian cancer is higher among women diagnosed with breast cancer at a younger age. In addition, the higher risk is limited to oestrogen-receptor-negative or oestrogen-receptor-unknown breast cancer [13]. Ovarian cancer risk is also higher in bowel cancer survivors diagnosed at a youn- ger age compared with women of the general population [73]. Heredity, radiation exposure, life style and reproductive factors may partially explain the high risk of ovarian cancer among breast cancer survivors. Ovarian cancer may link to breast and bowel cancers via BRCA1/2 mutations and Lynch syndrome or because of interactions with hormonal risk factors [13, 74]. History of prostate cancer in first-degree relatives was associated with a slightly raised risk of ovarian cancer (1.10, 95% CI: 1.01–1.20) compared with women with no family history of prostate cancer [75]. A 17% increase in ovarian cancer risk (95% CI: 1.03–1.34) was reported among siblings with a family history of prostate cancer compared with those with no family history [75].
Late menopause
The late age of natural menopause or the late-onset menopause begins by the age of 55 years and beyond. It is associated with a significant increase in the risk of ovarian cancer [14]. However, the elevated risk is observed only among cases with endometrioid and clear cell tumours [76]. Nevertheless, the total number of cycles during a woman’s life is significantly associated with ovarian cancer risk [77]. The age at the late onset of menopause carries intrinsic clinical and public health importance because the age at which natural menopause occurs may be a predictor of aging and health-related consequences (i.e. breast, ovarian and endometrial cancers).
Hormone replacement therapy
The relationship between ovarian cancer risk and HRT is ambiguous, as many studies have reported an increased risk of ovarian cancer among HRT users [16, 34, 78, 79], and the longer the duration of HRT use, the higher the risk [14], while others found no risk [80, 81]. A previous Danish study reported an increase of ovarian cancer risk among menopausal women using HRT regardless of duration of use, formulation, oestrogen dose, regimen, progestin type and administration route [79]. Women who sustainably used unopposed oestrogen replacement therapy had a 43% increase in epithelial ovarian cancer [OR = 1.43 (95% CI: 1.02–2.00)], and this finding is consistent with the outcome of other studies [16, 34, 78]. Although Purdie and Colleagues found comparable results [OR = 1.27 (95% CI: 0.86–1.88)], the use of unopposed oestrogen was associated with a three-fold increase in the risk of ovarian cancer when cases were compared with women with either hysterectomy or tubal ligation [OR = 3.00 (95% CI: 1.54–5.85)]. Furthermore, when the risk of ovarian cancer was studied by subtype, unopposed oestrogen replacement therapy was associated with more than a two-fold increase in risk of endometrioid or clear cell cancer [OR = 2.56 (95% CI: 1.32–4.94)]. The risk of endometrioid or clear cell cancer jumped to more than four-fold [OR = 4.29 (95% CI: 1.67–11.1)] when cases were compared with women with either hysterectomy or tubal ligation. In the same study every use of unopposed oestrogen provided 15% and 49% protection against mucinous and undifferentiated ovarian cancer tumours respectively [78].
Nulliparity
The risk of ovarian cancer is 24% higher among nul- liparous women compared with women who have one child. In addition, the risk increases more than 12-fold [OR = 12.5 (95%, CI: 2.4–63.80], when nulliparous women are compared with multiparous women [82]. Compared with women who have one child, nulliparous women have a 24% higher risk of ovarian cancer and the risk increases more than 12-fold. However, this increase in the risk of ovarian cancer varies significantly by tumour subtype. Nulliparous women had about a 50% higher risk of endometrioid ovarian cancers and about a 70% higher risk of clear cell cancers [35]. Most of the explanations for the relationship between childbearing and cancer have stressed the role of endocrine and metabolic factors.
Tobacco smoking
A meta-analysis study showed that the risk of mucinous ovarian cancer doubled among current smokers compared with controls, while smoking cessation returns the risk to normal in the long term [83], similar to what was observed later [16, 17]. Furthermore, current smokers have a 83–125% higher risk of ovarian mucinous borderline malignant tumours compared with never- smokers [16, 17]. In contrast, the risk of ovarian clear cell and ovarian endometrioid cancers was less than in never-smokers, while other types of ovarian cancer have no relationship with tobacco smoking [16, 17]. The variations in RR of ovarian cancer between different sub-types of ovarian cancer with regards to smoking raised a question of whether different ovarian cancer sub-types have different aetiology. Previous [84, 85] and most recent studies [86] have confirmed the inverse relationship between smoking and the risk of endometrial cancer. Endometrial cancer shares similar risk factors with ovarian cancer, including those related to the oestrogen window. Smoking reduces the age at menopause, dropping the number of menstrual cycles, thereby decreasing the carcinogenic effect of oestrogen [87]. Furthermore, tobacco smokers have lower endogenous oestrogen and/or higher androgen levels compared to non-smokers [88, 89]. Therefore, the type of ovarian cancer provoked by higher oestrogen levels must decrease among tobacco smokers, with the condition that other risk factors do not exist. In another words, tobacco smoking is a risk factor for mucinous tumours and protective against non-mucinous tumours.
Dietary fat
The prevalence of ovarian cancer is higher in western parts of Europe and Northern America compared to Mediterranean countries in Europe including Spain, Italy and Greece [15]. One explanation is that the diet in Mediterranean countries contains more fibre and more monounsaturated fats compared to the Westernised diet that contains more animal-based saturated fat and less fibre [15]. Diet could potentially influence the lifespan of the ovaries and sex hormone levels, and as a result affects the timing of natural menopause. Higher intakes of animal fat and cholesterol for a long period were significantly positively associated with risk of ovarian cancer in the Nurses’ Health Study [RR comparing extreme quartiles = 1.57 (95% CI: 1.20–2.06 and 1.35, 95% CI: 1.08–1.69), respectively] [20] in line with previously published data [90]. Compared with vegetarian diet consumers, an Indian study reported more than a six-fold increase in the risk of ovarian cancer among dietary fat consumers [19].
Controversial factors
Controversial factors include obesity, talc powder, radiotherapy, infertility, and fertility medications.
Obesity
The relationship between obesity and the risk of ovarian cancer is inconsistent as some studies reported positive association [21] while others reported no association [22]. The lack of consistency could be due to weakness in methodology (i.e. incorrect selection of controls or failure to control for potential confounders and/or inability to identify relevant confounders), small sample size, ethnic differences regarding the relationship with specific risk factors, and/or due to differences in epidemiological studies (i.e. case control vs. cohort studies). Obesity after menopause is differentially associated with higher breast cancer incidence, recurrence and death [91–93], while birth weight and obesity during childhood and adolescence have a positive association with ovarian cancer [91]. Nevertheless, no such positive correlation between adult obesity and ovarian cancer was detected [91], which is in line with other studies. The slight increase in ovarian cancer risk reported by some previous studies [21, 94, 95] was much clearer in case control studies than cohort studies [21]. It is well known that case control studies are prone to some limitations, including recall bias and inability to control for confounders [96]. Increased oestrogen level in obese women might link obesity to ovarian cancer, but among ovarian cancer sub-types only non-mucinous tumours are affected by oestrogen [97].
Talc powder
A prospective observational study that included more than 250,000 women from four large US-based studies, including The Nurses’ Health Study, The Nurses’ Health Study II, The Sister Study and The Women’s Health Initiative Observational Study showed no significant relationship between the use of talc powder in the genital area and risk of ovarian cancer among women [24], which is in agreement with previously published data [98]. Nevertheless, some previous studies have reported a positive link between talc powder and ovarian cancer suggesting possible carcinogenicity [23, 99, 100]. Furthermore, Cramer and colleagues reported that the relationship between genital talc use and the risk of ovarian cancer varies by histologic subtype, menopausal status at diagnosis, the use of hormone therapy, weight, and smoking [25]. Notably, studies that reported a positive association were case control studies [23, 99, 100]. Case control studies are prone to bias because they rely on recall, whereas this type of inaccuracy does not exist in prospective cohort studies. It is believed that talc particulates promote ovarian cancer development by disturbing the surface of epithelial tissue of the ovary when migrating through the reproductive channel [98]. However, the associations of combined powder use and ovarian cancer did not significantly differ according to tubal ligation status, suggesting a different mechanism [98].
Radiotherapy
The risk of ovarian cancer was found to be increased among women with breast cancer due to radiotherapy [101]. This finding was supported by some [26, 45] but not all the literature [102]. Ionizing radiation can damage the cell membrane and cell constituents including the DNA molecule, leading to genomic instability and promoting cancer development. Thus, the risk of a second cancer due to radiation depends on the dose and period of radiation delivered to the normal ovaries. A low dose of radiation (e.g. mammography, computed tomography scan) causes an insignificant increase in DNA damage and or chromosome aberrations. Such damage may activate signalling pathways responsible on DNA repair [103]. High radiation doses, used in treatment of certain cancer tumours, may cause irreversible damage in the DNA of healthy cells, and failure to repair such damage will initiate cancer development [104]. Similarly, women with other types of cancers (e.g. breast cancer, lung cancer) may have increased risk of developing ovarian cancer due to unbearable doses during radiation therapy [101]. Nevertheless, in women with BRCA1 and BRCA2 genes, there is still a great deal of controversy regarding the effects of low and high doses of radiation [105].
Infertility
Infertility is the inability of a woman to conceive after one year or longer of unprotected sex, and the cause of this problem is divided equally between man and woman. A previous study [29] reported a 60% increase in the risk of ovarian cancer in a cohort of infertile women [standardized incidence ratio = 1.6 (95% CI: 0.8–2.9)], which is in line with later studies [30]. It should be stated, however, that most studies in this field were unable to differentiate the underlying causes of infertility from the possible effects of fertility drugs. Most of the known causes of female infertility involved hormonal imbalances, which led to failure of ovulation (i.e. PCOS, amenorrhea or oligo-menorrhea). Other causes may include pelvic related factors such as endometriosis or tubal diseases. These causes of infertility may have an independent relationship with ovarian cancer.
The risk of a radiation-induced second cancer will depend on the dose delivered to each of the normal tissues.
Fertility medications
Although earlier studies [28, 31,106–109] had shown a significant increase in the risk of ovarian cancer among women who used fertility drugs and in vitro fertilization (IVF) technology (Table 2), later studies denied such an association [110–112]. Among women who used clomiphene citrate or gonadotropins, Brinton and colleagues reported that there is no increased risk of ovarian cancer [111]. This was confirmed even after the follow-up was extended to 30 years [32], in parallel with other findings [111, 112].
Table 2
Relationship between ovarian cancer and fertility drugs and in vitro fertilization in multiple studies
| Years and authors | Study type | Risk reported |
|---|---|---|
| Whittemore et al., 1992 [106] | Meta-analysis | There was a 2.8-fold increase in ovarian cancer risk among fertility drug users [OR = 2.8 (95% CI: 1.3–6.1] and the risk was 27-fold higher for infertile women who never got pregnant [OR = 27 (95% CI: 2.3–315.6)] |
| Rossing et al., 1994 [28] | Cohort | There was an eleven-fold increase in the risk of ovarian cancer in women with long-term use of clomiphene citrate (twelve or more cycles) [RR = 11.1 (95% CI: 1.5–82.3)], and it was detected in both women with persistent infertility and those who became pregnant, while no risk was detected for a period of less than one year of treatment |
| Sanner et al., 2009 [107] | Cohort | Gonadotropin treatment was associated with risk of invasive cancer [RR = 5.28 (95% CI: 1.70–16.47)] and for borderline tumours [SIR = 3.61 (95% CI: 1.45–7.44)]. The risk was increased seven-fold with clomiphene citrate treatment [SIR = 7.47 (95% CI: 1.54–21.83)] |
| Trabert et al., 2013 [32] | Cohort | Those who failed to get pregnant after extensive use of fertility drugs had more than a three-fold increase in ovarian cancer risk [RR = 3.63 (95% CI: 1. 36–9.72)] |
| Venn et al., 1995 [108] | Case control | Women with unexplained infertility who received IVF had more than a 19-fold increase in ovarian cancer risk [OR = 19.2 (95% CI: 2.2–165)] |
| Lerner-Geva et al., 2012 [31] | Cohort | The hazard ratio (HR) for ovarian cancer among women who received IVF was 58% [HR = 1.58 (95% CI: 0.75–3.29)] compared to controls |
| Rizzuto et al., 2013 [116] | Cohort | In subfertile treated women, the incidence of borderline tumours increased more than two-fold [SIR = 2.6 (95% CI: 1.4–4.6)] and to more than four-fold when the follow-up exceeded one year [HR = 4.23 (95% CI: 1.25–14.33)] |
| Stewart et al., 2013 B [109] | Cohort | There was a 76% increase in the risk of ovarian cancer among nulliparous women undergoing IVF and who remained nulliparous [HR = 1.76 (95% CI: 0.74–4.16)]. Women with endometriosis who remained nulliparous had a three-fold increase in the rate of ovarian cancer [HR = 3.11 (95% CI: 1.13–8.57)] |
The risk for borderline ovarian tumours was more than doubled [HR = 2.46 (95% CI: 1.20–5.04)] among women undergoing IVF [113], in parallel with an earlier study reporting a four-fold increase [114]. Women seeking fertility treatment may face one of four possible scenarios [87, 115]. There is a possibility that the interaction between the use of fertility medications, high concentrations of endogenous oestrogen and a high level of stress of treated women may trigger the development of ovarian cancer. Such a scenario is only applicable for infertile women who were exposed to higher doses of fertility medications/IVF for long periods and who had failed to get pregnant [115], in line with what was observed previously [32].
Women with BRCA1/2 mutations who were exposed to fertility medications and/or IVF treatments showed no risk increase regardless of treatment type [117]. Further, because most of these treatments successfully end with pregnancies and childbirth, they turn out to be protective against ovarian cancer. Therefore, women requiring fertility treatments should be reassured that fertility medications and/or IVF do not increase their ovarian cancer risk.
Conclusions
Ovarian cancer is the sixth most common cancer and the fifth most common cause of gynaecologic cancer deaths in women of developed countries. Ovarian cancer is asymptomatic and is not easy to detect by physical or laboratory examination until late stages, and this leads to a significant decrease in survival rate. Established risk factors for ovarian cancer include older age, genetic mutations, family history of breast and ovarian cancer, individual history of breast or other cancers, nulliparity, HRT and dietary fat. Tobacco smoking is associated with a significant reduction in oestrogen level; thus it has a protective effect against female cancers except for mucinous tumours, where it is considered as a risk factor. The relationship between obesity and ovarian cancer is inconsistent, although some detected a slight positive association between obesity and ovarian cancer risk, while others found no relationship. The relationship between infertility, talc powder, radiotherapy and the risk of ovarian cancer is controversial. Neither fertility medications nor IVF is associated with ovarian cancer risk. However, the risk may increase when infertile women are exposed to extensive fertility medications/IVF for long periods and never get pregnant. Increasing women’s awareness starts with education towards better understanding of the risk factors of ovarian cancer.











