INTRODUCTION
The transforming growth factor β (TGF-β), which, due to its pleiotropic pro-inflammatory and immunosuppressive effects, appears to be one of the cytokines involved in the processes of airway inflammation and remodeling, has attracted particular interest from researchers in the context of the pathogenesis of asthma and other allergic diseases. Apart from variable and reversible obstruction and bronchial hypersensitivity to specific and non-specific factors, inflammation and the resultant bronchial wall remodeling are the main pathophysiological processes in asthma. Elements of the immune system, such as T and B lymphocytes, antigen presenting cells (APCs), mast cells, macrophages, eosinophils, basophils, as well as structural elements of the bronchial tree, i.e., epithelial cells, vascular endothelial cells, fibroblasts, myofibroblasts, myocytes, nerve cells, are involved in chronic inflammation. One of the main immune mechanisms responsible for the initiation of inflammatory processes in asthma is imbalance between the Th1 and Th2 lymphocyte fraction, in favor of the latter. However, it has been increasingly observed, especially among patients with more severe forms of the disease, that Th1- and Th17-mediated inflammation also dominates. The inflammatory process, initiated by eosinophils, neutrophils, basophils, macrophages, mastocytes and the mediators they secrete into the airways, contributes to edema of the bronchial mucosa, excessive mucus secretion, goblet cell hyperplasia or bronchial smooth muscle contraction. In the case of a prolonged inflammatory process, there occur hyperplasia of fibroblasts and myofibroblasts, thickening of the epithelial basement membrane, subepithelial fibrosis, excessive proliferation of the extracellular matrix and promotion of angiogenesis [1–6]. The abovementioned processes of changes in the structure and stiffening of the airways are called remodeling, and TGF-β is believed to take an important part in the process. TGF-β belongs to the Transforming Growth Factor superfamily, and in the human body it is found in three isoforms (TGF-β 1-3). The TGF superfamily is divided into three subfamilies defined by similarity of protein sequences and signaling pathways – the first group, which includes TGF-β and activins, the second, which includes secretory protein synthesized in embryonic node cells (NODAL, nodal growth differentiation factor) and glial cell-derived neurotrophic factor (GDNF), and the third represented by bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs) and anti-Müllerian hormone (AMH). Depending on the type of tissue in which it is expressed, TGF-β regulates the processes of cell synthesis, proliferation, differentiation and apoptosis, and contributes to the maintenance of normal homeostasis of the body. It acts as an inhibitor of epidermal, endothelial, mesenchymal and some hematopoietic cells, while for chondroblasts, osteoblasts and neural tissue cells as a stimulator of growth and proliferation [7–10]. The TGF-β2 and TGF-β3 isoforms are important factors in the growth and regulation of neural cells, glial cells, microglia, astrocytes; they have an impact on neurotrophic factors and stimulators of neurite growth [11]. The studies conducted so far demonstrate that the main TGF-β isoform undergoing expression in the airways is TGF-β1 [7–9]. Its functions include inhibiting the proliferation and differentiation of the T and B lymphocytes, and NK cells, inhibiting the expression of MHC class II antigens, and participating in the proliferation and differentiation of structural cells of the bronchial tree such as fibroblasts and myofibroblasts. Apart from stimulating fibroblast proliferation, it also contributes to increased synthesis of collagen I and III, fibronectin, tenascin, proteoglycans, and inhibition of the action of collagenase and matrix metalloproteinases. Some researchers consider alveolar macrophages and lung fibroblasts to be the main source of TGF-β. Biopsy studies and bronchoalveolar lavage show an increased expression of this cytokine among asthmatic patients [8, 10–12]. The TGF-β2 isoform is less explored in terms of its action in the respiratory tract. It is primarily associated with specific roles it plays in morphogenesis processes of the respiratory, cardiovascular, skeletal and nervous system, as well as in regulation of the immune response and cell cycle through SMAD signaling pathways ((mothers against decapentaplegic homolog) – major signaling pathways of the TGF superfamily), stress-activated cascades, MAPK cascades (mitogen-activated N-terminal kinase) or phosphatidyl-inositol 3 kinases (PI3Ks) [11, 13–16]. The least studied isoform is TGF-β3, which is mostly expressed in mesenchymal tissues that affect proliferation and primary differentiation of internal organ cells. It has been postulated among some researchers that TGF-β3 along with TGF-β1, rather than alone, can induce the expression of fibroblast growth factors [17–19].
In our previous studies evaluating the role each specific TGF-β isoform plays in asthma, we observed significantly higher serum concentrations of TGF-β1 and TGF-β2 among patients compared to non-asthmatic individuals. As for the TGF-β3 cytokine, we did not find a statistically significant difference, only an upward trend. We also noted that TGF-β1 significantly correlated with TGF-β2, which could suggest a combined effect of the two cytokines in the inflammatory process in asthma. Although our study is in progress and still requires simultaneous comparison of serum TGF-β concentrations with the results obtained from more invasive studies (e.g., BAL, bronchoalveolar lavage), we can hypothesize that TGF-β plays an important role in the airways affected by asthma. Therefore, we thought it would be interesting to see if there are any correlations between concentrations of individual TGF-β isoforms and selected clinical features of asthmatic patients.
AIM
The aim of the present study was to evaluate the correlation of all TGF-β isoform serum levels (TGF-β 1–3) and clinical parameters (age of the participants, BMI and lung function parameters). A secondary objective was to assess an option of using TGF-β as a potential marker of airway remodeling and more severe asthma.
We hypothesize that higher concentrations of particular TGF-β isoforms may correlate with lung function parameters assessed by spirometry.
MATERIAL AND METHODS
The study included 69 subjects: 41 asthmatics and 28 non-asthmatic volunteers. The study participants were recruited from the Department of Allergology and the Allergy and Pulmonology Outpatient Clinic of N. Barlicki University Teaching Hospital No. 1 in Lodz, 90-153 Lodz, ul. Kopcinskiego 20.
Study group – inclusion criteria: written informed and voluntary consent to participate in the study, age over 18 years, documented history of asthma, asthma diagnosed according to the GINA guidelines, absence of other chronic respiratory diseases, accurate spirometry test giving an interpretable result, accurate skin prick test (SPT) giving an interpretable result, absence of diseases or medications that affect glucocorticosteroid metabolism.
Control group – inclusion criteria: written, informed and voluntary consent to participate in the study, age above 18 years, no history of respiratory diseases, no history of asthma, no history of any therapy with inhaled or systemic corticosteroids.
Exclusion criteria: the patient’s refusal to give consent at any stage of the study, age under 18 years, pregnancy and breastfeeding, clinical signs of exacerbation when being included in the study or in the preceding 4 weeks, history of a respiratory tract infection in the 4 weeks prior to the study.
STUDY PROTOCOL
The study was approved by the Bioethics Committee of the Medical University of Lodz (RNN/31/14/KE of February 11, 2014).
After providing full information about the study, voluntary written informed consent to participate in the research project was obtained from each subject. On the first appointment, medical interview and physical examination and spirometry were performed [13, 20, 21].
MOLECULAR AND BIOCHEMICAL ANALYSIS
Also, at the recruitment appointment, blood samples were taken from all the study participants for molecular-biochemical analysis (4 tubes – 2 EDTA-KE/9 ml tubes and 2 SERUM/9 ml tubes). In the patients subject to bronchial challenge testing (with a specific or non-specific agent), blood samples had been collected before the beginning of the procedure. The collected material was left for complete clotting for 30–60 min, and then centrifuged at 3000 rpm for 10 min using an MPW 223e centrifuge. The resulting serum was portioned into Eppendorf tubes and frozen at –20ºC. The serum was thawed at room temperature before testing. The Elabscience® Human TGF-β (TGF-β1, TGF-β2, TGF-β3) ELISA kit was used for determining serum protein concentration. Before use, reagents were prepared according to the manufacturer’s instructions, the serum was pre-warmed to 20°C and vortexed. Then, 30 μl of serum was added to 270 μl of Reagent Sample Diluent (1 : 10); next it was lightly vortexed and incubated in a water bath at 80°C for 8 min. After removal, the samples were cooled to 20°C for 5 min. All the reactants at room temperature were mixed thoroughly beforehand. Then, 100 μl of the mixture of serum and Reference Standard Sample Diluent (1 : 10) was transferred to the well, mixed thoroughly, and the plate was covered with the provided lid. It was then incubated at 37°C for 90 min. The solution was removed from the wells without washing, 100 μl of Biotinylated Detection Ab was administered immediately and the plate was covered with a sealer. After gentle mixing, they were incubated at 37°C for 60 min. Each well was then aspirated and washed three times with about 350 μl of Wash Buffer. After each step, all the solution was carefully removed from the well. After the last wash, the residual buffer was removed by aspiration. Then, 100 μl of HRP Conjugate was added to each well and covered with a sealer. They were incubated at 37°C for 30 min, and washed repeatedly five times. Next, 90 μl of Substrate Reagent was added to each well; they were covered with sealers and incubated at 37°C for 15 min. When a clear gradient was obtained, the reaction was stopped and 50 μl of Stop Solution was added to each well. After the color changed to yellow, reading was commenced with a plate reader preset to 450 nm and preheated.
STATISTICAL ANALYSIS
The database and statistical analysis were prepared using Statistica 13 software (TIBCO Software inc. 2017, Palo Alto, CA, USA). Selected descriptive statistics were presented using mean and standard deviation for continuous variables. Outliers were converted to limit values. Distribution analysis of continuous variables was made using the Shapiro-Wilk test to confirm normal distribution and the Levene’s test to check homogeneity of variance between the groups. The Student’s t-test for uncorrelated variables was used to compare the two groups, and the Mann-Whitney U test was used in the case of non-normality. Fisher’s exact test for “2x2” tables and a χ2 test with Pearson’s correction and Pearson’s correlation for continuous variables were used to evaluate the relationship between nominal variables. The analyses were performed for the whole study group and in the individual group, i.e., the asthmatic patients and the non-asthmatic participants. For all the tests used, α = 0.05 was considered as the level of significance (marked with an asterisk *). The results are presented in the tables and figures below, with statistically significant differences marked.
RESULTS
Both study subgroups (the control group – non-asthmatic subjects and the study group – the group of asthma patients) were balanced in terms of sex, body mass index (BMI), smoking and age (Table 1).
Table 1
Basic clinical parameters of the study and control groups
The performed analysis found a correlation between TGF-β1 and TGF-β2 levels and the age of the subjects. The older the subject, the more pronounced increase in TGF-β1 and TGF-β2 concentrations was observed.
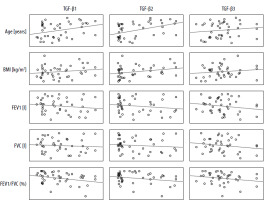
In the control group, statistically significant correlations were observed between TGF-β2 values and spirometric FEV1 and FVC values which were negative (p < 0.05). Additionally, TGF-β3 values and BMI were positively correlated with each other (p < 0.05) (Table 2, Figure 1).
Table 2
Pearson’s correlations between individual TGF-β isoforms and selected clinical parameters in the group of healthy subjects
Figure 1
Matrix of correlations between the TGF-β isoform serum levels [pg/ml] and clinical parameters in the control group
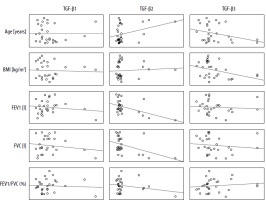
Positive correlations were observed between expression of TGF-β1 and TGF-β2 isoforms; both isoforms correlated with patient age. Correlations of TGF-β1, TGF-β2 and TGF-β3 expression with spirometric parameters regardless of the level of statistical significance had negative values (Table 3). The increase in TGF-β2 seems to follow two main patterns: dependent on other parameters (TGF-β1-dependent, reflected in worse spirometric parameters) – characteristic for the study group (patients) and independent (of TGF-β1 levels, not associated with worse spirometric parameters) (Figure 2) – characteristic for the control group (non-asthmatic individuals).
Table 3
Pearson’s correlations between individual TGF-β isoform serum levels and selected clinical parameters in the study group
DISCUSSION
Papers published widely in the recent years, focusing on identifying the mechanisms of inflammation and airway remodeling aim not only at a better understanding of the nature of the disease, but also at predicting it, preventing its progression and seeking individualized therapies. The TGF-β has attracted the attention of researchers because of its pleiotropism and widespread occurrence in the body, including the airways. Despite single reports casting doubt on the role of TGF-β on airway remodeling processes, a vast majority of papers show a markedly increased expression of specific TGF-β isoforms among asthmatic patients [8, 11, 14–16, 22, 23]. In our previous study, we proved that TGF-β1 and TGF-β2 isoform levels were statistically significantly higher among asthmatics compared to non-asthmatic volunteers. Therefore, in a follow-up study on this cytokine, we wanted to establish whether increased concentrations of particular isoforms could translate into clinical parameters in patients.
As regards the selected clinical characteristics, we observed a significant correlation between TGF-β concentrations and the age of the participants. TGF-β1 and TGF-β2 concentrations were higher in subjects over 40 years of age, but never in younger participants. What seems interesting is that this trend applied to all the study participants regardless of the division into groups, as well as to the patients and non-asthmatic participants separately. This may support the hypothesis that TGF-β plays a role in the aging processes. Numerous research studies have shown its overexpression in neurodegenerative diseases, Alzheimer’s disease, osteoarthritis, atherosclerosis or chronic kidney disease, i.e., conditions characteristic of old age [24]. Some of the research experiments proved that TGF-β, through its effect on activin type receptors (ActRII), ActRIIB, bone morphogenetic protein receptor (BMPR), anaplastic lymphoma kinase (ALK2), ALK3, ALK6, modulates the activation of transcriptional and post-transcriptional mechanisms of target genes, regulates cell proliferation and life cycle, including the processes of apoptosis and autophagy. It also regulates the processes of telomeric damage repair and unfolded protein response (UPR), crucial for aging, or finally – reactive oxygen species. The unfolded protein response is one of the main mechanisms for restoring cellular homeostasis by intensifying protein folding in the endoplasmic reticulum (ER) or by inducing apoptosis when equilibrium in the endoplasmic reticulum has not been achieved. It has been postulated that in fibroblasts, including airway fibroblasts, aging processes occur mainly through the UPR mechanism and senescence-associated secretory phenotype (SASP) [25, 26]. Some works also suggest that aging processes are associated with higher serum levels of pro-inflammatory cytokines, i.e., IL-1, IL-6, or just TGF-β. This is observed, for example, in the aging cardiac muscle, where increased fibrosis within fibroblasts and myocardial remodeling occur with age. In cardiology, TGF-β is considered a promising diagnostic and therapeutic target due to its effects on fibrosis and permanent remodeling of the heart after myocardial infarction, as well as in dilated and hypertrophic cardiomyopathies [27–29]. Researchers suggest that TGF-β’s effect on fibrosis is related not only to organs, but also to vascular smooth muscles. An increase in TGF-β expression also occurs in the central nervous system (CNS) in chronic damages, ischemia, Alzheimer’s disease, Parkinson’s disease or amyotrophic lateral sclerosis, where it promotes processes of fibrosis and neuronal loss [26, 30, 31]. Analyzing the above data, we can hypothesize that also in the respiratory system, higher TGF-β concentrations will translate into increased aging of fibroblasts, myocytes and myofibroblasts, promotion of fibrosis and inhibition of degradation of cellular matrix proteins. This, in turn, in the long term, may result in increased airway stiffness and clinically, perhaps, a poorer response to bronchodilators.
One of our hypotheses related to the effect increased TGF-β concentrations have on lung function parameters. In the course of our analysis, we demonstrated statistically significant negative correlations between TGF-β1 and FEV1%/FVC of the predicted value as well as between TGF-β2 and FEV1% of the predicted value, FVC of the predicted value and FEV1%/FVC of the predicted value. What seems interesting is that the correlations between TGF-β2 and the spirometric parameters listed above applied to all the study participants, not just the asthmatic patients. It is noteworthy that only the TGF-β2 isoform correlated with forced expiratory volume in the 1st s (FEV1), while the observed increase in TGF-β2 concentration was: 1) TGF-β1-dependent, occurring mainly in asthmatic subjects, in whom a significant decrease in spirometric values was also observed, and 2) TGF-β1-independent, occurring in non-asthmatic subjects, with no effect on spirometry.
The number of studies published to date and analyzing the relationship between cytokines of the TGF-β superfamily and lung function test values is negligible. The works of Panek et al. describe statistically significant differences between mRNA expression for TGF-β1 between groups of non-asthmatic individuals and patients with severe asthma, also based on spirometric criteria developed by the American Thoracic Society (ATS). A correlation was found only for FEV1 (%); other spirometric variables did not correlate significantly with ΔΔCT41. One of the publications we relied on to prove our hypotheses is the paper by Larkin et al. who indicated an increased ratio of TGF-β2/TGF-β1 mRNA expression in asthmatic patients, and a negative correlation between this index and FEV1 on baseline spirometry [32]. To support our results, we also refer to the paper by Gao et al. which, although focused on COPD patients, clearly shows an association between elevated TGF-β1 concentrations and significantly reduced FEV1 and FEV1%/FVC ratios on spirometry at each stage of the disease (A-E). What seems interesting about the aforementioned publication is that TGF-β1 concentrations are statistically significantly higher among patients with more advanced disease (C-E) [33, 34]. This observation may also be one of the arguments for the involvement of TGF-β in permanent airway remodeling. One of the main characteristics of COPD, especially its advanced form, is irreversible airway obstruction and excessive airway stiffness. Similar observations regarding TGF-β1 and COPD are described by De Boer et al. Their study shows that mRNA of receptors for TGF-β1 (TGF-β1R) and TGF-β1 protein levels in the bronchiolar and alveolar epithelium correlates significantly negatively with FEVI values [34]. Torrego et al. also tried to evaluate the relationship between TGF-β1 and TGF-β2 and spirometric values; however, they did not find an association between FEV1 decline in early or late allergen response and TGF-β1 and TGF-β2 expression in eosinophils and neutrophils after specific allergen provocation [35–37]. In the light of the few studies directly addressing the relationship between TGF-β and spirometric values, in my opinion, it is worth noting the inextricably linked phenomena of airway hyperresponsiveness (AHR) and remodeling. The mechanisms by which one phenomenon exacerbates the other, and vice versa, are not yet well explored. It has been postulated that TGF-β not only contributes to the structural remodeling of bronchial tree cells, but also exacerbates AHR by increasing the expression of myocyte contractile proteins. On the other hand, the contraction of myocytes and myofibroblasts induces TGF-β1 release, which can aggravate both bronchial hyperreactivity and remodeling [7, 38, 39]. Finally, since spirometry is not an entirely useful test for assessing airway remodeling, I wanted to highlight the study by Chae et al. They found that among asthmatic patients, the indices of bronchial wall thickness (WT) and wall area% (WA%), bronchial-to-arterial (BA) diameter, airway collapsibility (AC) and air trapping index (ATI) were correlated with FEV1%/FVC after bronchodilator administration, which the researchers suggest using as a potential surrogate marker of airway remodeling [40–42]. Yamaguchi et al., on the other hand, demonstrate that TGF-β levels in induced sputum correlates strongly positively with markers of bronchial wall thickness to body surface area (WA/BSA), while the WT/√BSA ratio correlates negatively with FEV1 values on spirometry in these patients. Considering other studies showing correlations between bronchial wall thickness on high-resolution computer tomography (HRCT) and the levels of TGF-β, matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMP), the main profibrotic cytokines in the sputum of induced asthmatics, it may be concluded that elevated TGF-β values may indirectly correspond to remodeling processes in the airways [43–45]. Nevertheless, to determine the usefulness of TGF-β for assessing bronchial remodeling, it would be necessary to perform simultaneous tests of serum TGF-β levels, its expression in the airways (e.g., airway epithelial biopsy studies, BAL, induced sputum), and spirometry and bronchial wall thickness by inspiratory-exhalation computed tomography. Despite the complexity and labor-intensive nature of the aforementioned studies, I believe that it is worthwhile to extend our knowledge of these two isoforms (TGF-β1 and TGF-β2) and their potential use as a biomarker of remodeling [14, 29, 40, 42, 43, 46]. The open question that remains is whether, as in the case of peripheral eosinophilia, determination of plasma concentrations of TGF-β and its isoforms could be useful, as an indirect marker of remodeling and prognosis of adverse forms of the disease, which is a goal to achieve of all asthma-treatment task forces all over the world [47, 48]. The search for readily available, non-invasive and inexpensive markers for predicting the course of chronic diseases is currently one of the main targets of researchers.
CONCLUSIONS
Concentrations of TGF-β1 and TGF-β2 isoforms increase with age. Statistically significant differences in the expression of both cytokines are observed as early as after the age of 40 years, which may not only prove the role TGF-β plays in the aging body, both among patients and non-asthmatic individuals, but also more intense bronchial remodeling processes occurring as the diseases progresses.
High TGF-β1 and TGF-β2 concentrations correlate with worse results of lung function parameters in patients, as assessed by FEV1%/FVC (TGF-β1 and TGF-β2) and FEV1 (TGF-β2).
TGF-β1 and TGF-β2 could be considered as an indirect potential marker of airway remodeling in bronchial asthma. However, this requires further studies based on assessment of serum and tissue TGF-β isoform concentrations, as well as spirometry and bronchial wall thickness indices in HRCT inspiration-exhalation.