Introduction
Drug-induced liver injury (DILI) is one of the most challenging liver disorders and gains increased attention annually, as it poses a significant risk to the patient’s health and because of the myriad of drugs used in clinical practice [1]. DILI broadly describes any injury to the liver that might occur because of medications (prescription or over the counter (OTC), herbal and dietary supplements (HDS), or other xenobiotics with hepatotoxic potential). It can present with a variety of clinical and pathological phenotypes that may range from asymptomatic liver test abnormalities to acute liver failure that cannot be attributed to other causes [2, 3].
The diagnosis is complex, with no unifying criteria requiring a high degree of awareness of the condition and careful exclusion of alternative etiologies of liver injury. Therefore, drugs that cause toxic effects on the liver exhibit diverse pathological responses that mimic all forms of acute and chronic hepatobiliary liver disease [4].
Drug-induced liver injury assessment has been organized into diagnostic scoring systems that are useful in organizing data into a categorical framework. However, they are not widely used in practice due to lack of popularity [5]. One of the most demanding and challenging issues of DILI is the attribution and assessment of causality. The Roussel Uclaf Causality Assessment Method (RUCAM) scale has been validated in several studies [6, 7].
Recognizing the pattern of liver injury at the initial presentation is vital. It provides a useful foundation to establish a differential diagnosis and guides the diagnostic evaluation accordingly. The R ratio is a quantitative expression of the injury pattern; it is defined as the ratio of serum alanine aminotransferase (ALT) to alkaline phosphatase (ALP) values, both expressed as multiples of the upper limit of normal (ULN), obtained at the onset of injury. An R ratio of > 5 indicates hepatocellular injury, < 2 indicates cholestatic injury, and 2–5 indicates mixed injury [8, 9].
A general approach to a suspected case of DILI includes a comprehensive medical and drug history, with clear timing regarding drug administration and exclusion of other potential factors that could contribute to the liver injury in addition to laboratory biomarkers including ALT [5].
The current blood biomarkers are suboptimal in detecting DILI and predicting its outcome. New alternatives are required to better diagnose and predict the DILI outcome. Glutamate dehydrogenase (GLDH) is a mitochondrial enzyme located in the centrilobular region of the liver, which plays a role in amino acid oxidation and urea production [10]. It is primarily found in the liver and to a lesser degree in the kidney, with trace amounts in skeletal muscle [11]. GLDH activity was thought to be a useful clinical indicator of the degree of liver damage added to the supposed ability of differentiation between toxic and viral hepatitis [12].
Aim
The aim of this study was to evaluate the diagnostic and prognostic role of GLDH and to perform a causality assessment in comparison to healthy controls and patients with acute viral hepatitis.
Material and methods
Study patients
This prospective study was conducted on one hundred subjects, in the Hepatology and Gastroenterology Department, National Liver Institute (NLI), Menoufia University, in the period from January 2019 to June 2020. Written informed consent approved by our institutional review board was provided by all enrolled patients (NLI IRB 000140). Patients were divided into two groups, with a healthy control group.
Group I: individuals without a history of liver disease or significant medical conditions, age 18 years or older, of both genders, and healthy lifestyle were included as a control group.
Group II: 40 patients with acute DILI were enrolled and the diagnosis was confirmed relying on a picture of acute liver injury based on levels of ALT more than 3-fold the upper limit of normal and/or alkaline phosphatase levels more than 2-fold the upper limit of normal. According to the R ratio of ALT to ALP (as a multiple of their upper normal limits): liver injury was defined as hepatocellular if > 5, mixed if 2–5, and cholestatic if < 2. Evident history of drug use within the past 3 months added to the causality, which was assessed by the Roussel Uclaf Causality Assessment Method, and liver biopsy if feasible was also a diagnostic prerequisite for DILI diagnosis after exclusion of all other causes of acute hepatitis [13, 14].
Group III: 40 other patients with acute viral hepatitis were enrolled with acute elevation of the liver function indices, in addition to serologic evidence of hepatotropic viral infection and exclusion of all other causes of acute hepatitis.
Exclusion criteria: any chronic liver disease – individuals with pre-existing chronic liver diseases (e.g., cirrhosis, chronic hepatitis); pregnancy and lactation – pregnant or lactating women; other causes of liver injury – individuals with liver injury due to causes other than drug-induced or viral hepatitis; concomitant serious illness – individuals with severe concomitant illnesses that may affect liver function; inability to provide informed consent – individuals unable or unwilling to provide informed consent for participation; history of substance abuse – individuals with a history of substance abuse or addiction; use of hepatotoxic drugs – individuals using known hepatotoxic drugs other than the ones under investigation; immunocompromised individuals – immunocompromised individuals, including those with HIV/AIDS or undergoing immunosuppressive therapy; individuals with unhealthy lifestyle; other exclusions – any other condition or situation that, in the opinion of the investigators, might compromise the study objectives.
Study procedures
Baseline demographic data including age, gender, residence, and occupation in addition to a detailed history with emphasis on drug history (type, timing, and dose) were recorded. Baseline laboratory indices such as ALT, aspartate aminotransferase (AST), serum bilirubin, fasting blood sugar, complete blood counts, prothrombin time and INR were measured. Also, serological tests for viral, autoimmune hepatitis and metabolic liver disease were performed.
Abdominal ultrasonography with doppler on portal and hepatic veins was done for all recruited patients and liver biopsy was done whenever eligible. The RUCAM was used to diagnose DILI. Only cases that had scored as highly probable or probable (more than or equal to 6 points according to RUCAM) were included [15, 16].
Glutamate dehydrogenase activity was assayed by ELISA technique. Serum samples were taken from all participants at first presentation to hospital and another sample was collected after 6-month follow-up. Serum samples were allowed to clot for 10–20 min at room temperature, then centrifugation at 2000–3000 RPM for 20 min was conducted and the supernatants were collected carefully. Serum samples were kept at 4°C for up to 72 h before aliquots were frozen at –80°C and stored until shipped for biomarker analysis. For all samples, GLDH was measured using SkanIt Software 3.1.0.4 RE for Multiskan FC with the research only kit (Bioassay Technology Laboratory). The detection range of the used kits is 0.1–35 U/l. The normal level of GLDH is ≤ 5.0 U/l in females and ≤ 7.0 U/l in males (glutamate).
Study outcome
The study outcome was to evaluate the diagnostic role of GLDH in patients with drug-induced liver injury in comparison to the control group and those with acute viral hepatitis, in an Egyptian cohort. All enrolled subjects were asked to return for repeated testing at 6 months thereafter for follow-up.
Statistical analyses
Descriptive statistics, such as means with SD, median with interquartile ranges and frequency distributions, were used to describe the cohort. Student’s t-test was used to compare means and SD of two sets of quantitative normally distributed data. The one-way ANOVA test was used to compare means and SD of > 2 sets of quantitative normally distributed data. The paired sample T test was used to compare means and SD of the same set of quantitative normally distributed data at different points of time, before and after treatment. The chi-squared test (χ2) was used to study the association between two quantitative variables. The ROC (receiver operating characteristic) curve was used for detection of sensitivity and specificity of GLDH level regarding diagnosis and prognosis of DILI. All statistical analyses were performed using the SPSS Statistics program version 23 for statistical analysis. A p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
In group I, males represented 45% and female 55%, with a mean age of 40.5 ±11.4 years. Males represented 25% and females 75%, with a mean age ±SD 46.5 ±10.4, in group II. All baseline laboratory measures are illustrated in Table I.
Table I
Baseline characteristics of the three studied groups
Criteria of incriminated drugs in DILI
Diclofenac was the most incriminated drug in the DILI group (16 patients, 40%), followed by amoxicillin clavulanic acid (N = 8, 20%), acetylsalicylic acid (N = 4, 10%), and others. Twenty-eight (70%) patients in the DILI group presented with hepatocellular injury, of which 14 (50%) cases were caused by diclofenac, 4 (14.2%) by acetylsalicylic acid, 4 (14.2%) by ibuprofen, 2(7.1%) by anti-rheumatoid treatment, 2 (7.1%) by SMX/TMP and 2 (7.1%) by amoxicillin clavulanic acid. The cholestatic pattern was present in 8 (20%) patients: 4 cases were caused (50%) by amoxicillin clavulanic acid, 2 (25%) by antiepileptic (carbamazepine) and 2 (25%) by an anabolic steroid. Four patients expressed mixed injury, of which 2 (50%) cases were caused by diclofenac and 2 (50%) by amoxicillin clavulanic acid. Diclofenac accounted for 66.6% (4 patients) of mortality cases, followed by anti-rheumatoid drugs and SMX/TMP (16.6%, 1 patient each), with a p-value of 0.28 (Table II).
Table II
Criteria of incriminated drugs in DILI group
GLDH studies
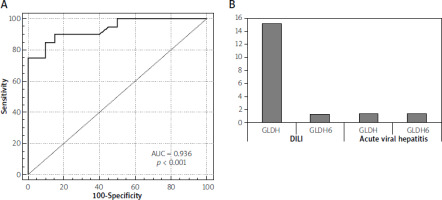
In our study, the mean value of GLDH was significantly higher in the DILI group (group II) than the healthy control (group I) and the acute viral hepatitis group (group III). The mean value ±SD was 18.5 ±10.4 U/l in the DILI group vs. 0.89 ±0.6 and 1.5 ±1.2 in group I and III, respectively (p-value < 0.001) (Table III). The mean value of GLDH in hepatocellular injury was 24.5 ±4.4 U/l, while it was 1.5.5 ±0.6 U/l in mixed type and 3.5 ±1.1 U/l in cholestatic injury (p < 0.001) (Table III). GLDH level showed a very strong correlation with ALT and AST levels and a poor correlation with total bilirubin level, while it showed a negative correlation with GGT and to lesser extent with alkaline phosphatase and prothrombin concentration (Table IV). The area under the curve for GLDH level was 0.936 with a p-value < 0.001 and cutoff point 2.1 U/l. GLDH showed 90% sensitivity, 85% specificity, 91.08% positive predictive value and 83.31% negative predictive value in the prediction of DILI (Figure 1 A). The mean value of GLDH level was significantly higher in the patients who died than in those who survived. It was 32.36 ±1.1 U/l vs. 15.36 ±10.1 U/l in the mortality group (p < 0.001). Follow-up of GLDH after 6 months improved (p = 0.012) (Figure 1 B). Multivariate analysis performed in this cohort group proved that middle age along with higher level of total bilirubin, and higher GLDH, are the predictors of poor outcome in the DILI group (Table V).
Table III
GLDH level in the different studied groups
Table IV
Correlation between GLDH and different laboratory biomarkers in the DILI group
| Liver biomarkers | GLDH | |
|---|---|---|
| R | P-value | |
| ALT | 0.840 | < 0.001 |
| AST | 0.710 | < 0.001 |
| ALP | –0.628 | < 0.001 |
| GGT | –0.167 | 0.300 |
| Total bilirubin | 0.317 | 0.055 |
| PC | –0.314 | 0.048 |
| Mortality | 0.852 | < 0.001 |
Table V
Multivariate analysis of predictors of poor outcome in DILI
Discussion
Drug-induced liver injury (DILI) is a rare but potentially severe clinical event caused by many types of prescription and non-prescription medications. It broadly describes any injury to the liver that might occur because of medications (prescription or over the counter – OTC), herbal and dietary supplements (HDS), or other xenobiotics [1, 2]. It is one of the leading causes of acute liver failure and it continues to be an important barrier to new drug development and marketing [17]. DILI remains a diagnosis of exclusion owing to the wide range of presentations and culprit agents with a potentially hepatotoxic effect. Thus, the diagnosis of DILI is based primarily on a detailed history and judicious use of blood tests, hepatobiliary imaging, and liver biopsy with careful assessment for other etiologies of liver disease before establishing a diagnosis [18, 19]. However, the diagnostic approach of DILI remains inaccurate because of a lack of reliable markers for use in general clinical practice. Definitely, the currently used liver parameters are neither specific nor able to distinguish DILI from other causes of liver injury or to predict the patient’s subsequent clinical outcome [20, 21]. Glutamate dehydrogenase (GLDH) is a relatively liver-specific enzyme expressed in the mitochondrial matrix of hepatocytes and is not altered in response to muscle injury when compared to ALT and AST [22, 23].
In the current study, regarding demographic data of the DILI group, the age ranged from 32 to 62 years, with a mean of 46.5 ±10.4 years (p = 0.002), and there was female predominance: 75% female vs. 25% male. In accordance with our study, 2009, in a study conducted in Spain where 603 cases of DILI were included, the overall mean age was 49 ±18 years among the recruited subjects, and older age has been proposed as a general risk factor for DILI [24]. However, the effect of age on DILI incidence was also paralleled by an increase in use of medications [25]. Moreover, the Spanish DILI registry showed that neither old age nor female sex was a predisposing factor for DILI, but age was an important predictor of clinical expression of hepatotoxicity [26]. Also, in the study conducted by Chalasani et al., 2015 and including 1257 subjects with suspected DILI, the mean age was 49 ±17 years, with only 16.6% of patients with DILI being 65 years old or older. This study showed a relatively equal sex distribution: 59% of patients with DILI were female [27]. Also, a study performed by Reuben et al., on 1198 subjects meeting criteria for acute liver failure, showed that among 133 cases of DILI assessed, 71% were female [28]. Generally, the explications of sex differences in the expression and severity of drug-related toxicity are considered hypothetical. Sensibly, it had been found that genetically determined impairment of the glutathione detoxification process, which determines the level of exposure to the reactive metabolite, occurred predominantly in women with DILI [27]. Secondly, the hepatotoxic effects of many drugs occur through autoimmune mechanisms, to which women are more vulnerable [29, 30].
In the current study, diclofenac was the most commonly incriminated drug, in 16 patients (40%), followed by amoxicillin-clavulanate (8 patients, 20%). Acetyl salicylic acid and ibuprofen were each responsible for 4 (10%) cases. In the study by Chalasani et al., antimicrobials were the most common class of causative drugs, accounting for 45%, followed by herbal agents and dietary supplements (HDS). Cardiovascular drugs accounted for 10%, central nervous system agents 9%, antineoplastic drugs 5%, and analgesics (largely nonsteroidal anti-inflammatory agents) 3% [27]. Moreover, Lucena et al., 2009, documented that antimicrobials and antiepileptics were the most common classes of implicated agents, followed by nonsteroidal anti-inflammatory drugs [26].
In our cohort, regarding survival, 34 (85%) patients showed complete recovery and normalization of liver functions after 6 months of follow-up. Neither chronicity nor liver transplantation cases were recorded. Six (15%) patients died from fulminant liver failure; all were female, with a mean age of 31.5 ±3.4 years (p < 0.002). Of the 6 cases of dead patients, 4 had taken diclofenac, 1 SMX/TMP, and in 1 case it was due to anti-rheumatoid treatment (sulfasalazine).
Alhaddad et al. reported a survival rate of 91.7%, with 1 case having undergone liver transplantation. They also documented that patients with amoxicillin clavulanic acid-induced DILI completely recovered from their injury. The mortality rate in diclofenac DILI cases was 12.9%, with complete recovery in 64.5% [30]. Chalasani et al. reported a 6% (56 patients) mortality rate among 899 patients with confirmed DILI [27]. Similarly, Reuben et al. conducted a prospective cohort study on 308 patients, and reported a mortality rate of 8%, with 2% requiring urgent liver transplantation [28]. In addition, Lucena et al. revealed that females showed the worst immediate outcome, with higher incidence of fulminant liver failure and liver transplantation [26].
In our study, hepatocellular injury was the dominant pattern, presented in 28 (70%) patients, followed by cholestatic variety in 8 (20%) patients and mixed injury in 4 (10%) patients (p = 0.003). Hepatocellular injury was the predominant liver injury pattern in the 6 patients who died. In the Chalasani et al. study, the pattern of liver injury was hepatocellular in 54%, and cholestatic or mixed in 23% each [27].
Bjornsson et al. reported a worse prognosis in patients with acute hepatocellular DILI than those with cholestatic or mixed liver injury pattern and that hepatocellular DILI has a high but variable mortality rate, depending on the drug involved [31].
There has been much effort to develop and qualify new liver-specific and safe biomarkers that outperform the current standard markers in terms of sensitivity, specificity and predictivity. In our study we examined the performance of GLDH in the diagnosis and prognosis prediction of DILI.
In the current study, regarding the detection of DILI, GLDH was specific to DILI cases compared to healthy volunteers and acute viral hepatitis groups with a mean value of 18.5 ±10.4 U/l in the DILI group vs. 0.89 ±0.6 U/l in controls, and 1.5 ±1.2 U/l in the viral hepatitis group respectively (p = 0.000). The area under the curve (AUC) was 0.936 (p < 0.001) with a cutoff point of 2.1. It showed 90% sensitivity, 85% specificity, 91.08% positive predictive value and 83.31% negative predictive value. It also showed a very strong correlation with ALT levels (r = 0.84, p < 0.001). In a study done by Church et al. to characterize the natural variability and performance characteristics of 14 different promising DILI biomarkers, GLDH correlated more closely with alanine aminotransferase than miR-122, with a value of r = 0.88 (p < 0.0001) for GLDH and r = 0.66 (p < 0.0001) for miR-122. GLDH and ccK18 had ROC AUCs > 0.90, suggesting that these biomarkers may be useful when screening for DILI [32]. Additionally, in a study conducted to evaluate the utility of glutamate dehydrogenase (GLDH) among other markers, as indicators of liver injury, GLDH had the highest diagnostic value, with an AUC of 0.98 [33].
Moreover, regarding the utility of GLDH as a prognostic marker of DILI, we found a statistically significantly higher level of GLDH in the patients who died vs. the survivors (32.36 ±1.1 vs. 15.36 ±10.1, p < 0.001).
The prognostic ability of GLDH may be attributed to the rapid elimination of GLDH following acetaminophen administration, while ALT levels remain elevated, and this is due to shorter half-life of GLDH when compared to ALT (~16 h vs. ~47 h, respectively), indicating that GLDH may serve as a “real-time” monitor for active or persistent liver injury [34].
Multivariate analysis performed in our cohort study revealed middle age (31.5 ±3.4 years) along with higher AST values (1622.5 ±443.1), higher ALT values (1889.5 ±385.1), higher level of total bilirubin (20 ±6.45), lower prothrombin concentrations (30.5 ±9.1%), and higher GLDH (32.36 ±1.1) to be predictors of poor DILI outcomes. Lucena et al., 2009, found that neither older age nor female gender was a predisposing factor for DILI, but that age, although not a modifiable risk factor, is an important predictor of clinical expression of hepatotoxicity. Older age (with an age cutoff of 60 years) is a determinant for the development of cholestatic damage with a male predominance, whereas younger age is associated with cytolytic damage and female overrepresentation. Mixed damage is independent of age [26].
Conclusions
The diagnostic approach of DILI is still rudimentary and inaccurate and requires a high index of suspicion, and thus careful assessment is required to distinguish DILI from other causes of liver injury. GLDH is highly specific to DILI cases and can serve as a potential screening test for early detection and outcome prediction.