Introduction
The number of hematopoietic stem cell transplantations (HSCTs) is constantly increasing and is currently over 1.4 million [1]. In 2021, the European Society for Blood and Marrow Transplantation reported over 47,000 procedures, with the predominance of autologous HSCT (auto-HSCT) (58%). The most common indication for the auto-HSCT procedure was plasma cell disorders (which includes multiple myeloma and others), constituting 55% of all auto-HSCTs, followed by non-Hodgkin’s lymphoma (NHL), constituting 26%, and Hodgkin lymphoma (HL), constituting 9% [2]. Over the last few decades, there has been a gradual increase in the number of patients diagnosed with lymphoma [3]. Approximately 30% of patients with NHL and 20% of patients with HL experience relapse after initial therapy [4, 5]. For relapsed/refractory (r/r) lymphoma, the prognosis of patients treated with conventional salvage regimens is unsatisfactory, so auto-HSCT preceded by high-dose chemotherapy has continued to be the mainstay of this treatment paradigm since the 1990s [6]. Nevertheless, the treatment of r/r lymphoma, as well as the choice of conditioning therapy, remains a topic of ongoing discussion.
The safety of the auto-HSCT procedure has improved over the years. However, this therapy is still associated with a number of potential side effects related to conditioning treatment, the most common of which are infections, mucositis, and hematologic toxicity [7]. Targeted approaches using checkpoint inhibitors, monoclonal antibodies, or potentially CAR-T therapy have filled an unmet need regarding the possibility of achieving clinically meaningful responses in r/r lymphomas [8]. These new drugs hold promise for changing peri-transplant management, as early inclusion of one or more of these agents may reduce the need for cytotoxic chemotherapy prior to auto-HSCT [9].
Historically, the carmustine, etoposide, cytarabine, and melphalan (BEAM) regimen was viewed as the standard of care for conditioning prior to auto-HSCT in patients with lymphoma. However, the toxicity of carmustine has led researchers to look for other options. One of them is bendamustine, which, combined with cytarabine, induces S-phase blockade and triggers apoptosis, enhancing the cytotoxic effect [10]. In further analyses, bendamustine has confirmed its safety and efficacy as an alternative to carmustine [11–14].
The aim of our study was to compare the adverse event profile between BEAM and Benda-EAM (BeEAM) regimens and to evaluate predictors and treatment outcomes in lymphoma patients undergoing auto-HSCT.
Material and methods
We present a single-center retrospective analysis of patients with lymphoma who received high-dose BEAM or BeEAM followed by auto-HSCT between January 2015 and December 2021 at the Department of Hematology and Transplantology in Lodz, Poland. We analyzed 82 patients (median age 52; IQR 38–62) with r/r HL (36.6%), mantle cell lymphoma (31.7%), or diffuse large B-cell lymphoma (18.3%) who underwent auto-HSCT after BEAM or BeEAM conditioning (47.6% and 52.4%, respectively). The study group characteristics are presented in Table 1. Data were collected from the electronic medical records.
Table 1
Patients’ characteristics
[i] ANC – absolute neutrophil count, auto-HSCT – autologous hematopoietic stem cell transplantation, CRP – C-reactive protein, DLBCL – diffuse large B-cell lymphoma, ECOG – Eastern Cooperative Oncology Group scale, Hb – hemoglobin, HCT-CI – hematopoietic cell transplantation-specific comorbidity index, HL – Hodgkin lymphoma, IQR – interquartile range, LDH – lactate dehydrogenase, MCL – mantle cell lymphoma, n – number, OS – overall survival, PFS – progression-free survival, PLT – platelets, UMW – Mann-Whitney U test, WBC – white blood cells
Patients received treatment according to BEAM following the standard operating procedure adopted at the clinic until 2018. At that time, the treatment strategy was changed to equivalent chemotherapy with bendamustine instead of carmustine because there were emerging data on the pulmonary toxicity of carmustine and it was a more expensive drug. Patients transplanted after 2018 were treated according only to the BeEAM regimen. During the COVID-19 pandemic, all patients were treated with bendamustine according to the center’s protocol. The pandemic did not affect the choice of treatment.
The BEAM conditioning regimen consisted of carmustine 300 mg/m2 on day –7, etoposide 150–200 mg/m2 twice a day (BID) on days –6 to –3, cytarabine 200 mg/m2/d BID on days –6 to –3, and melphalan 140 mg/m2 on day –2 In the BeEAM group carmustine was replaced by bendamustine, which was administered on days –8 and –7 at a dose of 160–200 mg/m2/day, while the other cytostatics were administered as in the BEAM protocol. All medications were administered intravenously.
All patients received granulocyte-colony stimulating factor at a dose of 300 µg for body weight < 60 kg and 480 µg for body weight ≥ 60 kg starting from day +3 after auto- HSCT until the absolute neutrophil count reached (ANC) 1 × 109/l for two consecutive days. Engraftment definitions were adopted from the Center for International Blood and Marrow Transplant Research (CIBMTR) Forms Manual: Post-TED. Toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE 5.0).
Prophylactic antimicrobial, antiviral, and antifungal treatment was applied in all patients from the beginning of chemotherapy to reaching ANC > 0.5 G/l. The prophylaxis for all patients consisted of ciprofloxacin 500 mg BID and fluconazole 400 mg once daily during the peritransplantation period; cotrimoxazole 960 mg three times a week from neutrophil recovery until six months after HSCT; acyclovir 800 mg BID during the peritransplantation period and after engraftment 200 mg three times a day for six months after HSCT.
In addition, all patients underwent environmental prophylaxis, manifesting with increased restriction of aseptic and antiseptic regimens in the Bone Marrow Transplantation Ward, including air-conditioned isolation rooms with high-efficiency particulate arrestance air, limited contact with visitors, and strict personal hygiene.
In all patients, a central vascular catheter was implanted before the transplantation procedure. Bacteremia was defined as a positive result of microbiological culture from a single sample or, in the case of Gram-positive bacteria infections, from a double blood sample, taken from a febrile patient. In the case of fever in patients with no clinically apparent signs of infection, lack of colonization with pathogens, and/or previous infection with a resistant pathogen, one of two empirical treatment options was used: cephalosporine with activity against Pseudomonas (cefepime or ceftazidime) or piperacillin with tazobactam. Patients with a complicated clinical course were administered carbapenem combined with glycopeptide/oxazolidine or β-lactam antibiotic acting against Pseudomonas together with aminoglycoside combined with glycopeptide/oxazolidine. In the case of a severe non-colonized condition, the patient was administered carbapenem together with aminoglycoside and glycopeptide/oxazolidine. The presence of colonization and/or a history of infection with a resistant pathogen were the reasons for implementing colonization-driven antibiotic therapy. The recommendations were modified according to the results of microbiological cultures and imaging examinations, and the treatment was continued for at least 72 hours after the fever and other symptoms of infection had subsided, and the granulocyte system (ANC > 0.5 G/l) had regenerated for two days. Patients with fever lasting more than 72–96 hours despite the introduction of broad-spectrum antibiotic therapy were administered an empirical antifungal treatment with the amphotericin B lipid complex or caspofungin [15]. The median duration of hospitalization from auto-HSCT was 22 days (range 10–44).
Statistical analysis
Qualitative parameters were compared using the χ2 test with Yates’s correction. Quantitative variables were compared using the Mann-Whitney U test or Student t-test, depending on the variable distribution. We assessed patient survival and time to engraftment probabilities through the Kaplan-Meier method and compared the two study groups using the log-rank test. Univariate and multivariate survival analyses were performed using the Cox proportional hazards method. In all analyses, p-values < 0.05 were considered significant. In the survival analysis, the confidence interval was set at 95%. Analyses were performed using Statistica Version 13 (StatSoft, Tulsa, OK) and MedCalc Software.
Results
Treatment outcome
A total of 82 patients with Hodgkin’s lymphoma or NHL receiving conditioning chemotherapy according to the BEAM (n = 39; 47.6%) or BeEAM (n = 43; 52.4%) regimen were included in the analysis. Both the baseline demographic and clinical characteristics of the two groups were comparable. No significant differences were found between variables such as type of 1st line chemotherapy regimen (p = 0.79) or number of lines of treatment preceding auto-HSCT (p = 0.42). The Deauville score before auto-HSCT did not differ between groups (p = 0.83). A comparable number of CD34+ cells was transplanted in both arms (median 4.1 × 106/kg in BEAM, and 3.6 × 106/kg in BeEAM, p = 0.19). A detailed description and comparison of the characteristics of each group are presented in Table 1.
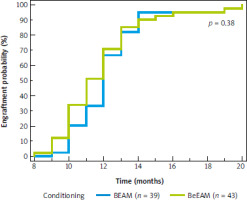
Median follow-up in the whole cohort was 50 months (95% CI: 37–59 months). The median overall survival (OS) since auto-HSCT for the whole cohort was 87 months (95% CI: 82–88 months); it was not achieved for the BEAM arm, and it was 82 months (95% CI: 73–82 months) for the BeEAM arm, p = 0.08 (Fig. 1). The median progression-free survival (PFS) since auto-HSCT for the whole cohort was 49 months (95% CI: 34–58 months), for BEAM and BeEAM groups 49 and 46 months, respectively (p = 0.56) (Fig. 2). Time to neutrophil engraftment was comparable in both BEAM and BeEAM groups; the median was 12 and 11 days, respectively (p = 0.38) (Fig. 3).
Fig. 1
Overall survival since autologous hematopoietic stem cell transplantation depending on conditioning regimen, log-rank test
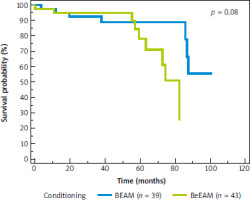
Fig. 2
Progression-free survival since autologous hematopoietic stem cell transplantation depending on conditioning regimen, log-rank test
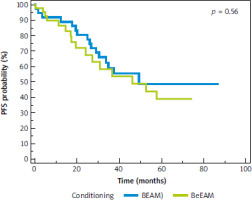
Fig. 3
Engraftment time since autologous hematopoietic stem cell transplantation depending on conditioning regimen, log-rank test
The early mortality rate (up to 100 days after transplant) was 3.7% (3 patients), including 2 patients in the bendamustine arm (4.7%), who died before full hematopoietic recovery, and 1 patient (2.6%) in the carmustine arm (death one month after auto-HSCT, already recovered). One of the fatal cases in the BeEAM arm was a patient who had experienced SARS-CoV-2-associated pneumonia several months before auto-HSCT and suffered a return of the virus infection during post-conditioning aplasia resulting in death due to respiratory failure. Transplantation was a form of salvage treatment in the active disease phase, as the patient had not achieved complete remission of his lymphoma. Another BeEAM patient died in the post-transplant period due to septic complications, and the one treated with BEAM died after hospital discharge due to pneumonia of undetermined etiology. Only one early death directly related to SARS-CoV-2 infection was reported.
Comparison of BEAM vs. BeEAM side effect profile
In the post-HSCT period, gastrointestinal side effects were the most common spectrum of complications, which occurred in 80.5% of patients as grade (G) ≥ 1 mucositis, significantly more frequently in the BeEAM than in the BEAM group (90.7% vs. 69.2%, respectively; p = 0.02). There was a higher incidence of severe (G3–4) mucositis in the BeEAM group; G3 was reported in 16% of patients, G4 in 7% of patients, while in the BEAM group G3 was reported in 10% with no G4 mucositis. 58% of patients experienced febrile neutropenia and its incidence was comparable between groups (51.3% vs. 64.3% in BEAM and BeEAM patients, respectively; p = 0.27). Bacteremia was found in 42.5% of cases, and again there was no difference between conditioning regimens (50.0% in BEAM vs. 35.7% in BeEAM; p = 0.26). Pneumonia occurred in 18.8% of patients and was significantly more common among patients treated with BEAM than BeEAM (31.6% vs. 7.1%; p = 0.01).
Patients in the bendamustine arm required significantly more platelet cell concentrate transfusions than patients receiving carmustine (median 20.5 vs. 15 (1 pack = 5 units or 1 unit from apheresis), p = 0.02). Regarding other hematological side effects, there were no differences between conditioning groups in the number of red cell concentrate transfusions, duration of G3 and 4 neutropenia, or G3 and 4 thrombocytopenia. We found that the number of transplanted CD34+ cells among patients in the entire cohort correlated negatively with the duration of G3 and 4 neutropenia (correlation coefficient: r = –0.29, p = 0.01 for G3 neutropenia; r = –0.28, p = 0.01 for G4 neutropenia). Number of days of hospitalization was similar in both groups (Table 2).
Table 2
Hematologic toxicity and treatment outcome
Evaluation of outcome predictors
In univariate Cox regression for OS, variables such as baseline C-reactive protein (CRP) level on the first day of hospitalization, hemoglobin (Hb) level at the time of auto-HSCT, number of days of G4 thrombocytopenia, and diagnosis of diffuse large B-cell lymphoma (DLBCL) showed an impact on survival. In addition, variables such as the number of days of G3 neutropenia and G4 thrombocytopenia, baseline Hb, and age had a significant effect on PFS in univariate analysis (Table 3). In the multivariate Cox regression model for OS, CRP on the first day of hospital stay (hazard ratio – HR = 1.03, 95% CI: 1.01–1.06) and days of G4 neutropenia (HR = 1.15, 95% CI: 1.00–1.32) were predictors of poorer survival, whereas Hb (HR = 0.43, 95% CI: 0.23–0.78) was a protective factor in terms of OS. Similarly, Hb (HR = 0.66, 95% CI: 0.45–0.96) was also a predictor of prolonged PFS (Table 4).
Table 3
Univariate analysis for overall survival and progression-free survival
[i] ANC – absolute neutrophil count, auto-HSCT – autologous hematopoietic stem cell transplantation, CRP – C-reactive protein, DLBCL – diffuse large B-cell lymphoma, Hb – hemoglobin, HL – Hodgkin lymphoma, HR – hazard ratio, LDH – lactate dehydrogenase, MCL – mantle cell lymphoma, OS – overall survival, PFS – progression-free survival
Table 4
Multivariate analysis for overall survival and progression-free survival
Discussion
Our study included a comprehensive comparative evaluation of treatment outcomes, predictive factors and adverse event profiles between BEAM and BeEAM regimens. As demonstrated in previous studies, we confirmed the lack of differences in PFS and OS between the two schemes. In an analysis conducted between 2011 and 2016, Frankiewicz et al. observed comparable probabilities of overall and PFS in both BEAM (n = 174) and BeEAM (n = 63) groups [16]. AlJohani et al. also detected no significant difference in median OS between groups (BEAM, n = 54; BeEAM, n = 17) [11]. Likewise, similar conclusions were reached in other centers [10, 17, 18]. In our study, 3 patients (3.7%; BeEAM – 4.7%; BEAM – 2.6%) experienced transplant-related mortality (TRM) during 100 days after auto-HSCT. These results are comparable to those presented by other researchers, where TRM for both BEAM and BeEAM conditioning is in the range 0–5% [14, 16, 19].
The BEAM and BeEAM conditioning regimens are associated with different toxicity profiles. Carmustine in the BEAM regimen can cause pulmonary toxicity, which is potentially related to the inhibition of glutathione reductase in alveolar macrophages [20, 21]. Our analysis revealed a significantly higher rate of pneumonia in patients treated with BEAM compared to BeEAM (31.6% vs. 7.1%; p = 0.01). These results are consistent with various studies analyzing treatment regimens using carmustine, in which pulmonary toxicity was observed in 5–63% of patients [14, 16, 21–23].
The high risk of pulmonary side effects has contributed to an active search for replacements for carmustine. Bendamustine has proven to be a less costly alternative, which moreover offsets the possibility of pulmonary toxicity [11]. However, the BeEAM regimen is associated with a higher incidence of mucositis and renal impairment [12, 24].
In our study, we found a significantly higher incidence of mucositis in the group of patients treated with BeEAM vs. BEAM (90.7% vs. 69.2%; p = 0.02). The prevalence of BeEAM-related mucositis varies widely across different studies, with rates ranging from 35 to 92% [11, 12, 14, 17, 19]. In a study performed on 87 patients, Garciaz et al. noted mucositis in 89% of BeEAM patients vs. 76% in the BEAM group [25]. Analysis conducted by Saleh et al. on 102 patients undergoing auto-HSCT between 2008–2015 revealed the presence of G3–4 mucositis in 50% of patients in the BeEAM group compared to 38% in the BEAM group [17].
Acute treatment-related renal impairment can be a complication in patients treated with BeEAM. However, these disorders are reversible in almost all patients, and transient hemodialysis is necessary only in selected cases [24]. In our study, we did not observe any significant acute kidney injury (AKI) requiring dialysis in the BeEAM group. These results are consistent with analysis by Frankiewicz et al., conducted on 237 patients (BeEAM, n = 63) in the period 2011–2016 [16]. However, in a study performed by Prediletto et al., in 122 lymphoma and myeloma patients receiving BeEAM between 2013 and 2016, definite rAKI (remaining on dialysis) was observed in 2.5% of patients [24]. The difference from our study may be due to the fact that in the above analysis, the total dose of bendamustine was used at a higher dose – 400 mg/m2.
The European Society for Blood and Marrow Transplantation analysis for the period 1980–2001 revealed a significant increase in the median time of 5-year survival after HSCT, related to a decreased number of lethal infectious complications [1, 26]. However, despite anti-infective prophylaxis, infections in the post-auto-HSCT period occur in 80–100% of patients [7, 27–29]. A variety of risk factors for infections after auto-HSCT have been defined, including duration and severity of neutropenia induced by treatment (< 7 vs. > 7 days; ANC < 0.5 G/l), virological status, and type of cancer [1, 30–32]. More than half of the infections responsible for TRM after auto-HSCT are associated with unspecified etiology. Meanwhile, of the known factors, infections of bacterial origin account for about 35%, fungal – 25–30%, viral – 20–30%, parasitic – 3–5%, and infections of mixed origin – 12% [7, 33]. Moreover, CIBMTR estimates that for auto-HSCT recipients, infections are responsible for 29% of deaths up to 100 days after HSCT, and for 5% in the late post-transplantation period [34]. In our study, patients who experienced TRM (3.7%) died of infection during 100 days after auto-HSCT. This observation is consistent with the findings of Frankiewicz et al., where in all cases the early mortality was caused by infections (3 cases in the BEAM group and 1 in the BeEAM group; p = 0.94) [16].
Our research revealed the presence of febrile neutropenia in 58% of all patients (BEAM – 51.3%; BeEAM – 64.3%), and bacteremia in 42.5% (BEAM – 50% vs. BeEAM – 35.7%), during the post-HSCT period, with no significant difference between BEAM and BeEAM conditioning. In our previous research, conducted in 2022 on 115 auto-HSCT recipients, bacteremia complicated the post-transplantation period in 43.5% of them, and febrile neutropenia was found in 77.4% [7]. Both studies by Garciaz et al. and Frankiewicz et al. also showed similar rates of infectious complications with BeEAM and BEAM conditioning [16, 25]. In the analysis conducted by Salazar et al., bacteremia was described in 31% of patients after auto-HSCT, while in the study conducted by Wang et al., the incidence of bacteremia reached 20% [35, 36]. As for neutropenic fever after auto-HSCT, the results vary widely depending on the underlying disease and the treatment used, usually ranging from 50 to 90% [7, 37, 38]. Our results are comparable to other published studies and confirm the lack of differences in infectious complications in patients receiving BeEAM vs. BEAM.
Moreover, we found that the higher number of transplanted CD34+ cells among patients in the entire cohort correlated with a reduction in the duration of G3–4 neutropenia, thus having a beneficial effect on effective neutrophil engraftment. Uysal et al. recently confirmed this observation in a study of 282 auto-HSCT recipients, which showed that the infusion of > 5 × 106/kg CD34+ stem cells may have a favorable effect on short-term outcomes of transplantation, including short-term neutrophil engraftment [39].
Although a significant proportion of patients can be cured with high-dose therapy with subsequent auto-HSCT, unfortunately up to 50% of these patients experience relapse [9]. Information on reliable and consistent predictors of outcome in lymphoma patients who undergo auto- HSCT is limited [40]. In our analysis, elevated CRP level on the first day of hospitalization and the duration of G4 neutropenia were predictors of worse survival. In contrast, initial higher Hb level was a protective factor for OS, as well as a predictor of prolonged PFS. Other predictors for the prognosis of auto-HSCT recipients, the importance of which is most frequently highlighted in the publications, include age, number of transplanted CD34+ cells, time to neutrophil recovery, disease status at transplant, negative 18F-fluoro-deoxy-d-glucose positron emission tomography status, lactate dehydrogenase and albumin level [41–43]. An analysis by Luo et al., which included 113 patients, revealed that low albumin level before transplantation was an independent risk factor in patients with lymphoma undergoing auto-HSCT [44]. Many other studies have confirmed that higher serum albumin levels at diagnosis are associated with better survival outcomes in patients with lymphoma, but they did not consider the auto- HSCT procedure [45–48]. In our study, we did not detect a significant effect of initial albumin level on the survival of auto-HSCT recipients. However, considering the unsatisfactory prognosis of patients with r/r lymphomas undergoing auto-HSCT, the search for prognostic factors remains an important issue. The co-occurrence of several risk factors in this group may help identify patients with a poor prognosis who may potentially benefit from novel agents in the pre- or post-transplant period.
Conclusions
Although this analysis has some limitations, including the retrospective nature of the study, the relatively small number of patients, and the predominance of patients with HL and mantle cell lymphoma (MCL), our data indicate that the BEAM and BeEAM regimens are comparably effective.
In terms of adverse effects, the BeEAM regimen has a more manageable toxicity profile, involving mainly mucositis and the need for more frequent transfusions. The risk of potentially fatal pulmonary toxicity with BEAM, on the other hand, creates the need for careful selection of patients with a history of pulmonary disease.
Simple laboratory variables, including Hb and CRP, may prove to be independent predictors of OS in auto-HSCT recipients. However, these observations need to be confirmed in studies on a larger group of patients.








