Introduction
The primary objective in the treatment of chronic hepatitis B is to improve survival by preventing progression of the disease and to provide protection against complications such as cirrhosis and hepatocellular carcinoma [1-3]. Interferons (IFNs) and nucleoside analogues are currently used in the treatment of chronic hepatitis B virus (HBV) infection [4]. In addition to their high antiviral activity that may reduce HBV-DNA to unmeasurable levels, entecavir (ETV), tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) from nucleoside analogues, which are considered to be a high barrier to HBV resistance, are also the only treatment option in decompensated cirrhosis, liver transplant patients, patients under immunosuppressive therapy, acute hepatitis B, extrahepatic manifestations of hepatitis B and in patients with previous experience of nucleoside analogues or who have developed resistance to nucleoside analogues [2, 5].
In 2001, TDF was the first antiviral nucleotide agent and also the first tenofovir to be approved by the Food and Drug Administration (FDA) for the treatment of human immunodeficiency virus (HIV) infection [6-8]. It was approved for the treatment of chronic hepatitis B in adults in 2008 [9]. Numerous case reports have linked the use of TDF with proximal tubulopathy, diabetes insipidus, decreased bone density, and impaired glomerular filtration. The majority of these published studies on renal safety or renal toxicity were conducted on HIV-infected patients because of its long term use [10-12]. On the other hand, TDF has been generally considered to be safe and well tolerated, because clinically important toxicities were rarely observed in clinical trials and case reports [2, 13]. While the mechanisms by which TDF causes renal toxicity can be explained by numerous pharmacokinetic and pharmacodynamic effects, the underlying mechanisms can be basically summarized as follows: mitochondrial DNA depletion, tubular cytotoxicity and intra-individual differences in TDF clearance because of polymorphisms in genes encoding for drug transporters. In addition to the basolateral membranes of the proximal tubular epithelial cells, provided with numerous mitochondria, TDF is involved in inactive cellular uptake by the organic anion transporters hOAT1 and hOAT3 so that TDF affinity for hOAT-1 and hOAT-3 is the basis of TDF nephrotoxicity [14, 15]. In the light of the possible mechanisms underlying the renal toxicity of TDF, TAF, again a prodrug called new tenofovir, has taken its place in daily use in the search for a drug as effective as TDF, but without side effects on renal and bone metabolism. Because of the fact that TAF is not a substrate for hOATs that inhibits the accumulation in the proximal tubular cells of the kidney unlike TDF it eliminates the risk of kidney injury due to OAT-induced cytotoxicity [16-18].In two registrational phase III studies comparing TAF with TDF in terms of efficacy and reliability, TAF was found to be superior to TDF in terms of renal functions with regard to both glomerular and tubular functions [19, 20]. In the light of these two studies, which also provided a reference to the recent guidelines, TAF and ETV have become the recommended nucleoside analogues in the management of hepatitis B for patients on hemodialysis and those who have undergone renal transplant [2, 5].
The management of chronic hepatitis B in hemodialysis patients and renal transplant recipients is of particular importance. Although ETV and TAF, a new tenofovir, are recommended by the guidelines based on the cohort or case-control analytical studies in this field, studies based on real life data, which are still limited in the literature, are evidently needed in this field.
In this study, we aimed to evaluate the reliability and efficacy of tenofovir disoproxil during long-term follow-up of hemodialysis patients and renal transplant recipients in our patient group based on real life data, which are limited in the literature.
Material and methods
Patients
This study was designed as a single-center, retrospective cohort study in the Department of Gastroenterohepatology, Istanbul Faculty of Medicine. Datareported here were collected retrospectively from outpatient visit charts between 2008 and 2012. The Ethics Committee of Istanbul University, Istanbul Medical Faculty approved the study protocol. Chronic hepatitis B (CHB) patients undergoing hemodialysis (group 1), renal transplant recipients (group 2) and patients with normal renal function (group 3) were included in the study. All patients signed the informed consent for this treatment.
Eligibility criteria for the study were as follows: (i) positive HBsAg for at least 12 months, (ii) HBV-DNA levels greater than 2000 IU/ml in serum for 6 months or more, (iii) elevation (at least 1.3× upper limit of normal) of serum alanine aminotransferase (ALT) levels for at least 3 months, (iv) biopsy proven chronic hepatitis (only for group 3), and (v) treatment with TDF for at least 6 months.
Exclusion criteria were as follows: (i) treatment with TDF < 6 months (ii) renal transplantation or hemodialysis period was < 6 months (iii) proven hepatocellular carcinoma.
Clinical assessments
All the other etiologies of chronic hepatitis were excluded in these patients. Antibodies to hepatitis C virus (anti-HCV) and total antibodies to hepatitis delta virus (anti-delta total) were also negative. Liver biopsy was performed at baseline in group 3 patients. Histologic changes [histologic activity index (HAI), and the extent of fibrosis] were assessed according to the Ishak scoring system [21]. Liver biopsy was performed in 2 patients who were undergoing hemodialysis and one renal transplanted patient. Child-Pugh scores were measured at baseline in patients with cirrhosis as previously described [22]. HBV-DNA levels were studied using the Cobas-TaqMan 96 system. All patients were treated with TDF for at least 6 months. HBV-DNA levels, biochemical parameters and clinical adverse effects were evaluated every 3 months. Symptoms that did not exist before treatment and were distinctly related with the start of the drug were recognized to be clinical drug adverse effects. TDF was initiated at a dosage of 245 mgonce a week after dialysis in group 1, and 245 mg/day in group 3. TDF dosage was adjusted according to the glomerular filtration rate in group 2 (creatinine clearence < 50 ml/min – TDF 245 mg every 24 hours; creatinine clearence 30-49 ml/min – TDF 245 mg every 48 hours; creatinine clearence 10-29 ml/min – TDF 245 mg every 72-96 hours).
Glomerular filtration rate (GFR) was calculated based on serum creatinine, sex, and age the using the Modification of Diet in Renal Disease (MDRD) formula [23]. The groups were compared with regards to safety and efficacy.
Statistical analysis
Statistical analyses were performed by using SPSS 15.0 statistical software package (SPSS Inc., Chicago, IL).Continuous variables are presented as means ± standard deviations or medians (ranges), while categorical variables are expressed as frequencies (percentages). Comparisons of continuous variables were performed by nonparametric Mann-Whitney U test when appropriate. A paired sample t test or a Wilcoxon test was used for comparisons of variables in paired samples. Differences between categorical variables were evaluated using the Pearson χ2 test or Fisher’s exact test when necessary. P-values less than 0.05 were considered statistically significant.
Results
Patients’ baseline features
A total of 217 patients with chronic hepatitis B (group 1: 8 patients, group 2: 9 patients, group 3: 200 patients) were enrolled in this study. Demographic and clinical features were similar in all groups (p > 0.05, Table 1). Two patients in group 1 and group 2 (11%) and 86 patients in group 3 (41.3%) were treatment-naïve (p = 0.001). Two patients in group 1 and group 2 (11%) and 44 patients (21.2%) in group 3 were cirrhotic (p = 0.311). Liver biopsy was performed in 4 patients in groups 1 and 2 (stages 1, 2, 3 and 6) and mean HAI was 6 ±1.4 (5-7). Liver fibrosis rates were as follows: in group 3, 30.8% stage 1, 34.3% stage 2, 19.6% stage 3, 11.2% stage 4, 1.4% stage 5, 0.7% stage 6. Mean HAI was 6.5 ±2.8 (2-16).
Table 1
Characteristics of patients
Virologic response
HBV-DNA negativity rates were comparable at the 12th month and at the end of the follow-up (50-83% for group 1, 60-67% for group 2 and 70-75% for group 3, p > 0.05).
Safety
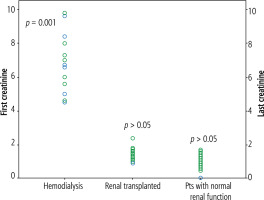
The frequency of clinical adverse effects (myalgia, nausea, headache, skin rash, insomnia, stomach ache, diarrhea) was significantly higher in groups 1 and 2compared with group 3 (37.5% vs. 11.1% vs. 0.5%, respectively, p < 0.001) (Table 2). However, no patients discontinued their treatment because of adverse effects. Serum creatinine levels were similar at baseline and at the end of the follow-up in groups 1 and 2 (6.5 ±1.8 mg/dl and 6.9 ±1.5 mg/dl; 1.3 ±0.2 and 1.4 ±0.4 mg/dl,respectively). GFR also did not change at the end of the follow-up period in the transplant and hemodialysis groups. On the other hand, serum creatinine level changed significantly over the course of treatment from a mean of 0.9 ±0.2 mg/dl (range, 0.5-1.5) at baseline to 0.9 ±0.2 mg/dl (range, 0.5-1.7) at the end of follow-up (p = 0.001) in group 3 (Fig. 1). GFR was significantly changed in patients who had normal renal function (Table 3). However, only 2 patients had creatinine levels > 1.5 mg/dl and 3 patients had an increase in serum creatinine of 0.5 mg/dl. No other biochemical parameters changed during the treatment. Serum phosphorus levels were similar in all groups before and at the end of the follow-up period (Table 4). No patients needed to adjust their TDF dosage interval.
Table 2
Adverse events
Table 3
Glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula
| Before treatment Mean (ml/min) | End of follow-up Mean (ml/min) | p | |
|---|---|---|---|
| Group 1 (n = 8) | 10 ±2.5 | 9 ±2 | 0.325 |
| Group 2 (n = 9) | 61.5 ±11 | 56.6 ±14 | 0.072 |
| Group 3 (n = 200) | 94.3 ±23 | 89.5 ±19 | 0.001 |
Table 4
Phosphorus levels of patients
| Before treatment Mean (mg/dl) | End of follow-up Mean (mg/dl) | p | |
|---|---|---|---|
| Group 1 (n = 8) | 5.6 ±1.2 | 5.2 ±1.3 | NS |
| Group 2 (n = 9) | 3.6 ±1.4 | 3.4 ±1.7 | NS |
| Group 3 (n = 200) | 3.8 ±1.1 | 3.7 ±1.3 | NS |
Two patients died of TDF at the 6th and 24th months in the hemodialysis group due to renal failure-related complications. HBV DNA was negative at the end of follow-up in both patients.
Discussion
The survival rates of renal transplant patients with HBV are worse than those of patients without HBV and the graft survival does not vary in this group of patients; the first cause of the mortality was found to be hepatic complications [24]. Similarly, hemodialysis patients with chronic HBV are known to have increased morbidity and mortality rates [2]. In the studies conducted, it has been found that antiviral therapy improves mortality [5]. Therefore, the requirement for an effective and safe treatment is of more importance for this particular group of patients. Tenofovir, which is safe and highly effective in the treatment of HBV-infected treatment-naive patients, is now a widely used antiviral agent in routine clinical practice; however, there are insufficient data on the safety of TDF in patients who have undergone renal transplant or those on hemodialysis.
In our study where we evaluated the efficacy and reliability of TDF in hemodialysis patients and renal transplant recipients, the group of patients whose treatment management is the most challenging, TDF was effective and well tolerated after long-term follow-up (median 24 months for groups 1 and 3, median 20 months for group 2) in these patients. In 8 hemodialysis patients, TDF was found to have no significant effect on GFR, creatinine or phosphorus levels. Despite the limited data on hemodialysis patients in the literature, Izzedine et al. [25] reported that the post-hemodialysis administration of 300 mg of TDF weekly was effective in terms of renal functions and providing HBV-DNA suppression in a 46-year-old cirrhotic-level male patient coinfected with HIV-1 and HBV who was undergoing hemodialysis due to polycystic kidney disease. In addition, Izzedine et al. calculated the ratio of the hemodialysis clearance of a drug as compared with its total body clearance in this case and indicated that tenofovir was dialyzable [25]. Kearney et al. [26] investigated patients with HIV seropositivity at different stages of liver and kidney disease to assess the pharmacokinetics, dose efficiency and suitability of TDF. Forty-one patients with a mean age of 56 years who participated in the study were classified according to creatinine clearances (CLCR) calculated in line with the Cockcroft-Gault equation as follows: patients with normal renal function (CLCR > 80 ml/min) 3 people, those with mild dysfunction (CLCR 50-79 ml/min) 10 people, those with moderate dysfunction (CLCR 30-49 ml/min) 8 people, those with severe dysfunction (CLCR 10-29 ml/min) 11 people, those with end-stage renal disease (ESRD) requiring hemodialysis 9 people. As in our study, they found that there was no worsening in the creatinine values of the patients with severe dysfunction and undergoing hemodialysis compared to the patients classified according to creatinine clearances. Since tenofovir clearance was also decreased in parallel with renal functions, they emphasized that TDF was effective and safe with dose adjustment in these patients [26]. Aleman et al. [27] added tenofovir disoproxil so as to be 245 mg once weekly to the treatment with abacavir and ritonavir since flare-up was detected in HBV-DNA due to lamivudine resistance in a 46-year-old patient coinfected with HIV-1 and HBV and with ESRD due to HIV-associated nephropathy who was receiving peritoneal dialysis for four days a week. When they evaluated the drug levels of tenofovir in the peritoneal fluid and serum during the follow-ups of this patient, they found the drug level above the therapeutic range, and since they saw tenofovir being partially excreted by peritoneal dialysis, they adjusted the dose of tenofovir disoproxil so as to be 245 mg every two weeks. They stated that HBV-DNA was effectively suppressed and no side effects were observed during the patient’s follow-ups [27].
In addition to hemodialysis patients, treatment of chronic HBV is of importance in renal transplant patients as well. In our study, we evaluated the efficacy of TDF in 9 renal transplant patients monoinfected with HBV who had treatment experience. We found that it was effective and safe in terms of both HBV-DNA suppression and renal functions that we evaluated with GFR and serum creatinine levels. Although there are limited data in the literature in this regard, in one of the studies published, Daude et al. [28] reported 3 renal transplant patients treated with tenofovir. None of the patients had acute rejection or renal dysfunction similar to our patients [28]. In a case report published by Battaglia et al. [29], a 58-year-old male patient with type 2 diabetes mellitus (DM), arterial hypertension and comorbid chronic HBV, whose antiviral treatment was converted to TDF due to flare-up in HBV-DNA levels when he was on entecavir, was evaluated. HBV-DNA was significantly suppressed about 8 months after converting the treatment to TDF; kidney biopsy was performed on the patient, who was found to have a decrease in GFR level and an increase in proteinuria, and no acute graft rejection was detected. During the 5th month follow-up of the patient, whose TDF dose was adjusted according to the GFR and immunosuppressive treatment was changed, HBV-DNA was found to be negative, while renal functions were preserved and proteinuria regressed. It has been reported that TDF was effective in suppressing HBV-DNA in renal transplant patients and can be safely used with renal function monitoring [28]. Because the use of immunosuppressive drugs and comorbidities such as DM in the etiology of renal disease, arterial HT and rheumatologic diseases continue to pose a risk to the kidney in the post-transplant period, it is rather difficult to analyze in isolation the efficacy and safety of TDF in renal transplant patients with a risk of potential nephrotoxicity. Therefore, real-life data play an important role in the challenges we face in patient management.
Tenofovir is one of the most popular nucleoside analogues in the treatment of HBV because it presents a high barrier in terms of efficacy and resistance. With the effective use of TAF in our day, the risk of nephrotoxicity is one of the main handicaps of TDF, which we can describe as either the first or the old tenofovir. In a randomized clinical trial by Marcellin et al. [29] conducted on patients with normal renal functions and HBV, they followed up 426 patients using TDF for 144 weeks in terms of renal functions. In that study, a 0.5 mg/dl increase was observed in creatinine levels in 0.5% of the patients compared to the pre-treatment period, and it was found that GFR levels did not decrease to < 50 ml/min in any patient [29]. In our study, the mean GFR levels did not decrease to < 50 ml/min after a mean follow-up of 26 ±10 months in patients with normal GFR levels (94.3 ±23 ml/min) at the beginning of the follow-up period, similar to the study of Marcellin et al. [29]; however, the decrease in GFR was observed to be higher compared to that of the hemodialysis and renal transplant patient group. One of the reasons for this may be the longer duration of TDF use in this group. Moreover, because of the retrospective nature of the study, the fact that it may have effects on renal functions as a limiting characteristic and that data such as body mass index and comorbid disease records could not be evaluated has reduced our scope of interpretation. In our study, another limitation is that we did not use new markers such as neutrophil gelatinase associated lipocalin, which can detect tubular injury earlier and more effectively [14]. Additionally, the potential drug resistance and type of past therapy of patients, classification of the phases of chronic hepatitis B, the concentration of sugar and proteins in urine, and the possible changes in the mineral density of bones in densitometry were missing data of our study that can be listed as limitations of the study. Jung et al. [8] followed up a total of 110 chronic HBV patients without previously known kidney disease (creatinine < 1.5 mg/dl, GFR > 60 ml/min) for a mean period of 103 weeks. They found a significant reduction in GFR levels after 96 weeks (92.05 ±1.63) compared to the mean baseline (106.35 ±1.07 ml/min). They emphasized that advanced age, presence of DM and bilirubin levels were effective in TDF’s effect on renal functions [8].
Our study showed that the clinical adverse effects (e.g. myalgia, nausea, and headache) of TDF were more common in patients with CRF compared to patients without CRF. However, the occurrence of adverse effects does not necessarily require discontinuation of the drug. In fact, the occurrence of adverse effects in this group of patients was an expected result since hemodialysis patients are open to both hemodynamic and metabolic variations.
In conclusion, clinical adverse effects of TDF were more common in patients with CRF compared to patients without CRF. However, the occurrence of adverse effects did not necessarily require discontinuation of the drug. TDF was well tolerated and effective for this group of patients. GFR did not change at the end of the follow-up period in the renal transplant or hemodialysis group. Moreover, our patients did not have acute rejection or renal dysfunction. However, there was a significant change in the GFR of the patients with normal renal functions at the end of the follow-up period (94.3 ±23 vs. 89.5 ±19, p < 0.001). Regardless, none of the patients required adjustment of their TDF dose with regard to GFR. The virologic response rate was also similar in all groups.
We found that TDF was well tolerated and effective in our study where we evaluated the reliability and efficacy of tenofovir disoproxil during long-term follow-up of hemodialysis patients and renal transplant recipients, whose management is challenging and who require a careful follow-up, in our patient group in line with real-life data.