Introduction
Neuroendocrine tumours (NETs) arise from endocrine cells located in various parts of the body. Over two-thirds of NETs derive from the gastrointestinal tract (gastro-entero-pancreatic neuroendocrine tumours, GEPNETs), followed by bronchopulmonary NETs (BPNETs) [1, 2]. The incidence of NETs has increased over the last decades. According to studies performed in the USA (the Surveilance, Epidemiology, and End Results [SEER] Program), the incidence increased from 1.09/100,000 in 1973 to 6.98/100,000 in 2012 [3].
Based on Ki-67 proliferation index, the 2019 World Health Organisation (WHO) classification divided GEP-NETs into the following categories: well differentiated NETs G1 (Ki-67 < 3%), G2 (Ki-67 from 3–20%) and G3 (Ki-67 > 20), and poorly differentiated small- and large-cell neuroendocrine cancers (NECs) (Ki-67 > 20%) [4]. According to the 2021 WHO classification of lung tumours, broncho-pulmonary neuroendocrine tumours (BPNETs) are divided into 4 groups: typical carcinoids/neuroendocrine tumours grade 1, atypical carcinoids/neuroendocrine tumours grade 2, large cell neuroendocrine carcinomas, and small cell carcinomas [5].
The unique feature of NETs is their ability to produce and secrete hormones and biogenic amines, leading to distinctive clinical symptoms (functional tumours). Nevertheless, most of the tumours are non-functioning [2, 6], which leads to late diagnosis at the advanced stage and worse prognosis [7, 8].
Therefore, there is a need to look for an efficient and accessible diagnostic tool for these tumours. Advanced imaging and molecular methods are still not generally available and generate high cost to healthcare systems. Hence, we tried to find an accessible diagnostic marker for neuroendocrine tumours. Tumour-promoting inflammation is one of the hallmarks of cancer [9]; thus, in our study we evaluated circulating interleukin 6 (IL-6), oncostatin M (OSM), and cardiotrophin-1 (CT1).
Interleukin 6 is a potent pleiotropic cytokine that regulates cell growth and differentiation and plays an important role in the immune response. Dysregulated production of IL-6 and its receptor are implicated in the pathogenesis of many diseases, such as multiple myeloma, autoimmune diseases, and prostate cancer [10]. Elevated IL-6 has been considered a poor prognostic marker in patients with cholangiocarcinoma (CCA). Interleukin 6 secreted by primary cancer- associated fibroblasts is capable of inhibiting autophagy in CCA cells, thereby stimulating their proliferation [11, 12].
Oncostatin M is a member of the IL-6 family [13]. It is considered to play multiple roles inter alia in cell differentiation, inflammation, haematopoiesis, and metabolism [13–15]. It has been identified as a driver of protumourigenic signals and is associated with poor prognosis in some cancer types such as pancreatic [16] and gastric cancers [17], breast cancer [18], or glioblastoma [19]. Oncostatin M induces IL-6 chemokines [13, 16].
Another member of the IL-6 superfamily, CT1, is expressed in may tissues and organs, such as heart, liver, kidneys, or lungs [20]. Cardiotrophin-1 is known to be associated with cardiovascular diseases. It triggers inflammatory and atherogenic molecule expression and stimulates cell proliferation, migration, and apoptosis [21–23]. Akkoc et al. showed CT1 as a potent regulator of cancer-associated fibroblast autophagy in breast cancer [24].
Every member of the IL-6 family orchestrates critical responses in the physiological regulation of development, metabolism, haematopoiesis, and inflammation. Consequently, aberrant activity of these cytokines frequently contributes to the pathogenesis of cancer [14]. Knowing the utility of the mentioned cytokines in many types of cancer, in our study we tried to assess serum levels in neuroendocrine tumours originating from the digestive tract and lungs.
Material and methods
All patients were recruited at the Department of Endocrinology and Neuroendocrine Tumours, Medical University of Silesia. Informed written consent was obtained from all study participants.
Cohorts
The study assessed 142 individuals. Eighty (56%) comprised the study group, which consisted of several subgroups including gastroenteropancreatic neuroendocrine tumours (GEPNETs, n = 64, 80%): pancreatic (PNETs, n = 24, 30%), small intestine ileal/jejunal (SINETs, n = 21, 26%), duodenal (DNETs, n = 4, 5%), gastric (GNETs, n = 8, 10%), rectal (RNETs, n = 7, 9%), and lung neuroendocrine tumours (BPNETs, n = 16; 20%); typical carcinoids (n = 10, 13%); and atypical carcinoids (n = 6, 7%). Following a review of medical records, data pertaining to patients’ neuroendocrine markers, specifically chromogranin A, serotonin, and 5-HIAA, was collected.
The control group comprised 62 healthy volunteers. All controls were asymptomatic with no history of any malignancy at the time of blood draw. Table 1 shows a qualitative and quantitative comparison of the study and control groups.
Table 1
Characteristics of the study participants
The main inclusion criterion for the study group was a histologically confirmed diagnosis of neuroendocrine tumour. Exclusion criteria for both groups were pregnancy, lactation, unsure medical family history, age less than 18 years, chronic or acute heart, liver, or renal failure, and lack of informed consent to participate in the study.
Sample collection
Peripheral blood samples were collected and clotted. Sera were obtained by centrifugation (1500 rpm/15 min) and stored at –80°C until assayed. The serum concentrations of IL-6, OSM, and CT1 were measured using the Enzyme-Linked Immunosorbent Assay Kits (ELISA, Cloud-Clone Corp., TX, USA). The procedures were done according to the instruction manual Cat Nos. SEA079Hu for IL-6, SEA110Hu for OSM, and SEA810Hu for CT1.
The analytical procedure was in accordance with the regulations given by the manufacturer in the technological manuals attached to the set. To determine the concentrations of the tested samples, a calibration curve was prepared using the standards included in the kit. Absorbance readings at 450 nm were performed using a mikroQuant reader (Bio Tek World Headquarters, California, USA), while the processing of the results was carried out using the KCJunior program (Bio Tek, USA). Kit sensitivity – the minimum detectable dose of IL-6 is usually less than 2.9 pg/ml, OSM is usually lower than 6.3 pg/ml, and CT1 is usually lower than 6.5 pg/ml. For all kits the intra-series error was < 10% and the extra-series error was < 12%. All samples were run in duplicate. Results were reported as concentration pg/ml. All ELISAs tests were performed at the Department of Medical and Molecular Biology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia in Katowice.
Radiological evaluation and clinical staging
Disease extent was assessed using anatomical computed tomography (CT) and/or magnetic resonance imaging and functional imaging ([68Ga]Ga-DOTATATE positron emission tomography [PET]/CT) in well-differentiated NETs and ([18F]FDG PET/CT) in G2/G3 NETs. Images were evaluated by an experienced specialist in radiology and/or nuclear medicine. Gastroscopy and colonoscopy were utilised for assessment of GNETs and RNETs.
Histological diagnosis
All NET patients had histologically confirmed neuroendocrine tumour disease reported by an experienced pathologist in accordance with WHO 2019 (for GEPNETs) and WHO 2015 (for BPNETs). Specimens were evaluated by H&E staining and immunohistochemistry.
Statistical analysis
The normality of distributions was assessed using the Shapiro-Wilk test. Data for normal distributions are presented as mean values ± standard deviation (m ±s), and for skewed distributions as medians (lower-upper quarters) – Me (Q1–Q3). Comparisons between study group and control group for patients’ characteristics were performed using Student’s t-test or Mann-Whitney U test. For assessing the relationship between variables, the Spearman rank correlation coefficient (rS) was calculated.
Two-tailed tests were used, and the significance level was set at p < 0.05. Statistical analysis was performed using Statistica 13 (TIBCO Software Inc., 2017; http://statistica.io).
Results
Characteristics of the cohorts
Demographic and biochemical characteristics of the study participants are shown in Table 1. The study group consisted of 64 GEPNET patients (80%) and 16 BPNET patients (20%). Eighty-three of the participants were female (58%). The median age of the study group was 55 years, range 22–81 years. The median body mass index (BMI) was 25.5 kg/m2, range 22.8–28.7. Forty-four per cent of the study cohort had advanced disease. The most common metastasis site was lymph nodes (34%), followed by liver (25%), and bones (9%). Fourteen per cent of the patients had functional disease. Complete clinical data of the study cohort is shown in Table 2.
Table 2
Clinical data of the study cohort
There were no statistically significant differences between the study and control groups regarding sex (p = 0.343), age (p = 0.083), BMI (p = 0.385), and smoking habits (p = 0.911). A significant difference between the groups was noted in the occurrence of diabetes mellitus (p < 0.05). However, there were no significant differences in the occurrence of hypertension (p = 0.205).
Interleukin 6, oncostatin M, and cardiotrophin-1
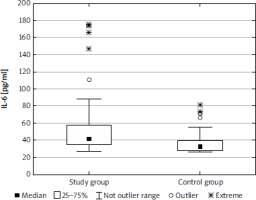
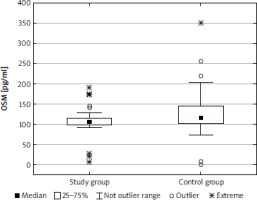
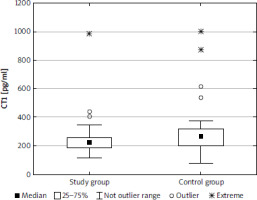
The median (lower-upper quartile) concentration of IL-6 was 41.5 (34.8–57.9) pg/ml in the study group and 32.6 (27.9–39.2) pg/ml in the control group, and the difference was statistically significant (p < 0.001) (Fig. 1). The concentration of OSM was significantly lower in the study group than in the control group (p < 0.001): 105.6 (98.1–114.7) pg/ml and 115.5 (102.6–145.6) pg/ml, respectively (Fig. 2). There was a significant difference (p < 0.01) in concentration of CT1 in the study group (222.0 [186.6–258.7] pg/ml) and controls (267.2 [199.1–316.1] pg/ml) (Fig. 3). No correlation between the concentrations of IL-1, OSM, and CT1 depending on age, BMI, and Ki-67 index were observed. There was no correlation between IL-6 and OSM concentration and clinical stage; however, a weak negative correlation between CT1 concentration and clinical stage was observed (p < 0.05) in the study cohort (Table 3). There were no differences between IL-6, OSM, and CT1 levels at the primary site both in the BP-NET and the GEP-NET group (Table 4). In the study group there were no statistically significant differences for smokers in the levels of IL-6 (z = 0.499, p = 0.618), OSM (z = 1.317, p = 0.188), and CT1 (z = 0.458, p = 0.647). No correlation was noted between commonly used NET markers, including serum chromogranin A level, serum serotonin level, or urine 5-hydroxyindoleacetic acid (5-HIAA) concentration and the serum levels of IL-6, OSM, and CT1 (Table 5). There was no correlation between IL-6 and OSM (rS = 0.015, p = 0.859) and CT1 (rS = 0.179, p = 0.121). Also, there was no correlation between OSM and CT1 (rS = –0.139, p = 0.220).
Table 3
Correlation between body mass index, age, Ki-67, staging, and the measured parameters for the study and control groups
Table 4
Concentration of parameters related with primary site
Table 5
Correlation between parameters related with concentrations of known neuroendocrine tumour markers
Discussion
In recent years, there has been growing interest in exploring biomarkers for early diagnosis and prognostic assessment of tumours. Among these biomarkers, circulating IL-6 and its family have shown promise [10–12]. This study aimed to evaluate the potential of these factors in the diagnosis of GEPNETs and BPNETs.
The interleukin 6 cytokine family, including IL-6, OSM, and CT1, are critical players linking inflammation and cancer. These cytokines signal through receptors that share the glycoprotein 130 (gp130) subunit. Upon ligand binding, they activate Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathways, particularly STAT3, which promotes cancer cell proliferation, survival, and metastasis. Notably, IL-6 and OSM have been demonstrated to play pro-tumourigenic roles [13].
The study by Geisler et al. showed that IL-6 concentration may correspond with histopathological grading in NET – patients with G2 tumours displayed significantly lower IL-6 levels compared to patients with G1 NETs. Additionally, the results pointed out higher IL-6 levels in NET patients than in healthy controls [25], and this corresponds with our study. We observed a statistically significant difference between circulating IL-6 median concentration in the study group vs. the control group (p < 0.001). Another paper evaluated the influence of metabolic syndrome on inflammatory pathways in patients with well-differentiated NETs, revealing higher IL-6 expression in the peritumoural area of GEPNETs in patients with progressive disease [26].
High levels of IL-6 have been associated with poor prognosis and shorter survival in various cancer types, while lower levels are linked with better therapy response in cancers such as multiple myeloma, non-small cell lung carcinoma, oesophageal carcinoma, colorectal carcinoma, renal cell carcinoma, prostate carcinoma, breast carcinoma, and ovarian carcinoma [27–29]. Magidey-Klein et al. demonstrated that IL-6 influences the metastatic process through IL-6/IL-6Ra signalling, which mediates the differentiation of haematopoietic stem and progenitor cells into premetastatic monocytes-dendritic progenitors that functionally differentiate into immunosuppressive monocytes and support the metastatic switch [30]. Research indicates that IL-6 induces programmed death ligand 1 (PD-L1) expression and promotes tumour growth, e.g. in glioblastoma, hepatocellular carcinoma, lung cancer, or pancreatic cancer [13, 31–35].
All IL-6 family cytokines transmit signals through the JAK-STAT pathway, activating a diverse range of signalling pathways and transcription factors. They stimulate epithelial-mesenchymal transition processes, promoting the development of mesenchymal traits in cancer cells [31]. Inhibition of this cytokine has been shown to improve immunotherapy results. Interleukin 6-related cytokine blockage can have an antitumour effect by influencing the tumour microenvironment [13]. Oncostatin M modulates the microenvironment of the tumour and plays a role in cancer progression. Recent evidence suggests that OSM promotes tumour growth and relates to poor prognosis in several types of malignancies including pancreatic cancer, gastric cancer, breast cancer, glioblastoma, and cervical cancer [16, 17, 36–38]. Within cancer cells, OSM promotes malignancy by triggering processes such as epithelial-mesenchymal transition, the acquisition of cancer stem cell-like characteristics, migration, invasion, and the enhancement of tumourigenic and metastatic potential [19, 31, 39–41]. Studies revealed that CT1 is overexpressed in some cancer cells and can induce fibroblast autophagy, resulting in cancer cell migration and metastasis [24, 42]. It can induce ovarian carcinoma cell proliferation [43]. Another study pointed out the connection between CT1 and liver metastases in colon cancer [44]. Our analysis showed a weak negative correlation between CT1 concentration and clinical stage in the study cohort (p < 0.05). Nonetheless, we observed significantly lower levels of circulating OSM (p < 0.001) and CT1 (p < 0.01) in the study group compared to the control group. We assume that this outcome may relate to the stable phase of the disease with no signs of progression. These results confirm the complexity of the processes of carcinogenesis and metastasis. Growth and progression of tumours rely on a complex microenvironment comprising inter alia diverse cell types, stromal components, and a specific array of cytokines and chemokines. Despite the pivotal role of this microenvironment in tumour development, there are few detailed data on the characteristics of the tumour microenvironment in the context of neuroendocrine tumours, leaving the role of the tumour immune microenvironment in these tumours unclear [45, 46]. As shown in previous studies of other malignancies, OSM and CT1 relate to tumour growth and advancement [16, 17, 19, 24, 31, 36–44]. Xue et al. showed that inhibiting OSM by certain long noncoding RNA transcripts restrains the tumourigenesis and metastasis in GEPNETs [47].
Inflammation in tumourigenesis is still not fully ascertained and is still in the field of research. The progressive development of immunotherapy in recent years has led to an increase in interest in new targets. Interleukin 6 cytokines influence cancer cells mostly as tumour promoters within the tumour microenvironment [13]. We believe our study may show potential in evaluating serum cytokines in NETs; however, we know it has limitations due to its retrospective, single-centred nature and relatively small, diverse patient cohort. Furthermore, our study cohort consisted of individuals diagnosed with well-differentiated neuroendocrine tumours. Despite the presence of advanced disease in some cases, there was no evidence of disease progression. We speculate that this lack of disease progression may account for the observed concentration of OSM and CT1 in the study group.
While existing evidence suggests its association with tumour development and progression, further studies are warranted to validate diagnostic utility of IL-6 and elucidate their underlying mechanisms in NET pathogenesis. On the other hand, low levels of OSM and CT1 could be a tool for assessing and/or confirming disease stabilisation. A comprehensive understanding of the role of cytokines in NETs may facilitate early diagnosis, prognostic assessment, and personalised treatment strategies. Given that NETs have diverse phenotypes influenced by tumour origin, identifying a fair immunological biomarker presents a challenge. Therefore, we recognise the necessity for additional and more comprehensive investigations to elucidate the significance of inflammatory cytokines in the progression of neuroendocrine tumours on a broader scale.
Conclusions
In conclusion, our investigation into selected IL-6 family cytokines revealed differential modulation of signal transduction pathways. These findings suggest that despite utilising a common signalling transducer, individual IL-6 family cytokines exert distinct biological effects on NET development. Notably, IL-6 appears to promote tumourigenesis, while OSM and CT1 exhibit inhibitory effects gastro-entero-pancreatic and bronchial neuroendocrine tumour development.