Introduction
Lung cancer is the malignant neoplasm with the highest mortality rate worldwide. Achieving better treatment outcome depends on detection of early stages of the disease, in the pursuit of more efficacious treatment modalities or clinical conditions which are considered favorable prognostic factors in this group of patients. Recent scientific advances have enabled the creation of new molecule-based immunotherapy, although the benefits of non-specific immunotherapy in cancer treatment were observed as early as in the 1960s [1–8]. Improved overall survival in patients who had postoperative pleural empyema (PPE) due to lung cancer surgery was described [2, 4, 9]. BCG immunotherapy in this group of patients was attempted [10–12]. Benefits of PPE were reported in patients who underwent either lobe or an entire lung resection. However, some reports do not confirm a similar relationship [2–4, 13–16]. Pneumonectomy combined with a maximal scope of mediastinal lymphadenectomy is the most extensive single surgery available to cancer patients, enabling standardized treatment outcome comparisons [3–5, 17, 18]. The postoperative empty space after pneumonectomy is the biggest in the human body and may be prone to chronic inflammation of the non-specific type of immunostimulation. Its recurrent nature makes further assessment easier [3, 4, 8, 15, 16].
Aim
We aim to compare the survival outcomes of patients with non-small-cell lung carcinoma (NSCLC) who developed PPE after pneumonectomy with uneventful recoveries available in our center’s database.
Material and methods
Patients
From 1995 to 2009, 928 pneumonectomies were performed in the authors’ center, which is on average 62 per year. The PPE syndrome occurred in 39 cases (39/928, 4.2% of total pneumonectomies), with a broncho-pleural fistula (BPF) rate of 1.8% (16/928) [4]. One patient died, and two other patients ended up with permanent chest tubes. Out of the 36 patients who had recovered from PPE, 34 were diagnosed with NSCLC. The other 2 patients were non-oncological. The NSCLC group was extended by a patient with an infiltrating type of carcinoid tumor, similar in clinical presentation to less aggressive types of cancer [19]. Two cases of mixed tumor were excluded from further analysis due to lack of comparative data. The study group consisted of 32 patients who underwent surgery due to NSCLC and developed postpneumonectomy empyema. Postpneumonectomy empyema occurred in 15 right-sided cases and 17 left-sided cases with BPF frequency 6 right/8 left. The control group comprised 96 patients who were treated with pneumonectomy due to NSCLC (with an analogous case of carcinoid tumor) and had uneventful recoveries. Cases of unknown survival outcome that were not subject to follow-up were also eliminated from comparisons. The selection of the control group took into account the requirements for propensity score matching in terms of age, sex, tumor histopathology, TNM classification (6th edition) and the side of surgery (Table I). The compared groups were recruited by ratio as follows: T3N0 squamous carcinoma (ca) left side, male – 5 : 19, T3N0 squamous ca right side, male – 4 : 10, T2N2 squamous ca left side, male – 2 : 8, T3N0 adeno ca left side, male – 2 : 6, T3N2 squamous ca left side, male – 2 : 5, T3N0 adeno ca right side, male – 2 : 4, T3N1 squamous ca left side, male – 1 : 6, T2N0 squamous ca right side, male – 1 : 5, T3N0 squamous ca left side, female – 1 : 5, T4N2 adeno ca left side, female – 1 : 5, T2N2 squamous ca right side, male – 1 : 4, T4N0 squamous ca left side, male – 1 : 4, T3N2 adeno ca left side, male – 1 : 3, T2N2 adeno ca right side, male – 1 : 2, T2N0 squamous ca left side, male – 1 : 2, T3N2 adeno ca right side, male – 1 : 2, T3N1 adeno ca right side, male – 1 : 2. The matching of groups T3N1 squamous ca right side, female and T3N0 carcinoid right side, male was possible for recruitment only in equal ratio of 1 : 1, while the matching of groups T3N0 squamous ca right side, female was possible for recruitment only in equal ratio 2 : 2 as well. The ratio of cases in the study and control group on the left side was 17 : 63 (1/3.7), and on the right side 15 : 33 (1/2.2) as well. The age category was selected according to the nearest neighbor method with age difference not exceeding 10 years (radius matching method), according to the final rule (32/96) – 1 : 3 without return for the whole groups. T4 cases in both groups involved limited left atrial infiltration with the possibility of R0 surgery and the groups examined did not include cases of a giant cell carcinoma. The groups did not differ statistically significantly in terms of proportions of traits analyzed: age – p = 0.38, sex – p = 0.78, histopathology – p = 0.61, value of T – p = 0.84, and N – p = 0.95, staging – p = 0.99, side of operation – p = 0.2. Adjuvant therapy was implemented in approximately 60% of cases in both groups (Table I).
Table I
Characteristics of groups with and without PPE
Surgery
All patients had originally presented for lung surgery via thoracotomy under general anesthesia, with the placement of a double-lumen endotracheal tube. The bronchial stump was closed by means of manual suture according to Klinkenberg or a linear stapler with the reinforcement of mediastinal adipose tissue. The scope of right lymphadenectomy comprised nodes from groups 4R, 7, 8, 9R, 10R, and in selected cases from groups 2R, 3a and 3p. On the left side, dissected lymph nodes were from groups 5, 6, 7, 8, 9L, 10L, and in selected cases from group 4L. T4 and N2, stages IIIA and IIIB were an indication for neoadjuvant or adjuvant therapy (Table I). All cases were assessed based on the 6th edition of the TNM system [7].
Postpneumonectomy empyema
The acute phase of empyema involves accumulation of purulent fluid in the pleural cavity. In this exudative phase the volume of the thoracic cavity remains unchanged and both pleural membranes remain flexible. After 4 weeks, empyema enters a chronic phase, which is associated with transformation of the purulent fluid into granulation tissue. It leads to thickening of the parietal and visceral pleura. Furthermore, the volume of the thoracic cavity decreases. Table II summarizes treatment methods in patients with PPE. In 43.75% (14/32) of cases the cause of PPE formation was a bronchial stump fistula. Two fistulas were closed surgically by means of myoplastic techniques, whereas 12 fistulas were closed endoscopically by the application of glue or repeated application of 20% silver nitrate in the chronic phase of empyema. Acute PPE was treated with passive drainage, repeated punctures, or both. Procedural treatment was administered in the chronic phase of PPE, on average on the 418.9th day of empyema (41–5205). Twenty-seven (84.4%) PPE patients were cured solely by accelerated treatment (AT), 4 (12.5%) by additional partial thoracomyoplastic surgery, and 1 (3.1%) by supplementary chest fenestration. The applied AT method in the treatment of PPE in most cases relies on threefold revision of the pleural cavity (every 48 h) with repeated debridement by means of curettage and intensive lavage of the empyema cavity with 10% povidone-iodine solution. In 2 cases myoplastic surgery of the bronchial fistula was performed in the first stage of the procedure. The first two stages are completed by filling the cavity with povidone-iodine-soaked towels with temporary chest closure [8, 9, 13, 14]. In the final stage of the procedure the pleural cavity is filled with antibiotic solution and definitively closed.
Table II
Characteristics of treatment of the entire PPE-group
Statistical analysis
Survival analysis was conducted based on the stratification according to overall deaths, cancer-related and cancer-unrelated deaths in both groups examined separately and jointly. The influence of particular factors (sex, age, side of surgery, T and N value, clinical staging, histological type of tumor) on death risk was determined. The point of reference for reviewed survival outcomes was the day pneumonectomy was performed. Cancer-related and cancer-unrelated deaths as well as survival outcomes and the number of patients alive in both groups were juxtaposed. Statistical analysis of survival was carried out according to Kaplan-Meier curves, the log-rank test, the univariate and multivariate Cox proportional hazards model, Fisher’s exact test, the chi-square (χ2) test, and the Mann-Whitney U test. The p-value less than 0.05 (p < 0.05) was set as a statistically significant threshold for the results obtained.
Results
The results obtained were analyzed in reference to outcomes in the management of empyema and those in the management of cancer. In all 32 patients permanent resolution of the PPE symptom was achieved. No deaths during hospitalization were recorded in the case group. Assessment of the oncological outcomes was presented by contrasting with the outcomes achieved in the group who underwent pneumonectomy with uneventful recoveries. Both groups were examined in terms of total follow-up period, and the number of overall, cancer-related and cancer-unrelated deaths. Average and median survival was assessed, and survival outcomes were compared (Table III). The follow-up period in both groups indicates that patients were treated simultaneously. Analysis of the remaining parameters indicates superiority of survival outcomes in the PPE group over the group with uneventful recoveries.
Table III
Juxtaposition of survival outcomes in groups with and without PPE
Recovering from PPE correlated with excellent survival outcomes. Predicted 5- and 10-year survival rates were 71% and 59%, respectively, even though none of the patients was in the 1st stage of diagnosis (Figure 1) [18]. Average and median survival were almost 2-fold and 3-fold longer in the PPE group. The percentage of survivors at the time of analysis in the PPE group was 2-fold higher than in the group without PPE. In the PPE group, the number of patients with a 5-, 7- and 10-year follow-up period was almost 2-, 2.5-, and 3.5-fold higher than in the group with uneventful recoveries, respectively. A 2-fold lower percentage of cancer-related deaths was noted in the PPE group compared to the group without PPE (15.63% vs. 32.29%, respectively). However, in the comparison of cancer-unrelated deaths a reverse trend was observed. There was a 1.5-fold higher percentage of deaths in the group with a PPE complication in comparison with the group without empyema (37.5% vs. 23.95 %) Nevertheless, the above comparison is insufficient because the cause of death was impossible to determine in 21 (21.88%) patients from the group with uneventful recoveries. A statistically significant difference in survival rates was found in favor of the PPE group for overall deaths (p < 0.001) and for cancer-related deaths (p = 0.003). In contrast, a similar dependence was not recorded for cancer-unrelated deaths (p = 0.63) (Table III, Figures 1–3).
Figure 1
Kaplan-Meier survival curves for overall deaths. Yes – group with PPE. No – group without PPE (log-rank test p < 0.001)
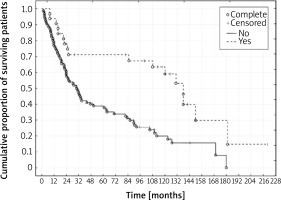
Figure 2
Kaplan-Meier survival curves for cancer-related deaths. Yes – group with PPE. No – group without PPE (log-rank test p = 0.003)
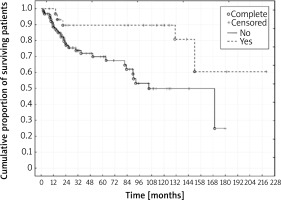
Figure 3
Kaplan-Meier survival curves for cancer-unrelated deaths. Yes – group with PPE. No – group without PPE (log-rank test p = 0.63)

In the multivariate model for the PPE group, which included sex, age, descriptors T and N as covariates, female sex (HR = 0.26, 95% CI: 0.07–0.94, p = 0.04) and higher T stage (HR = 0.26, 95% CI: 0.07–0.94, p = 0.04) were statistically significant independent factors for overall death risk. The very same set of covariates influenced cancer-related deaths (females – HR = 0.04, 95% CI: 0.003–0.81, p = 0.03), although the analogous influence of the T descriptor was on the threshold of statistical significance (HR = 40.97, 95% CI: 0.92–1808.46, p = 0.054). In the PPE group, a significant influence of progression of the N stage on death risk was not proven (HR = 1.26, 95% CI: 0.7–2.3, p = 0.43 – overall deaths; HR = 3.78, 95% CI: 0.84–17.03, p = 0.08 – cancer-related deaths; HR = 0.86, 95% CI: 0.38–1.93, p = 0.73 – cancer-unrelated deaths). Older age did not statistically influence on cancer-unrelated death risk (HR = 1.09, 95% CI: 0.99–1.21, p = 0.06). Neither sex (HR = 0.38, 95% CI: 0.07–1.99, p = 0.25), nor progression of the T stage (HR = 2.78, 95% CI: 0.62–12.46, p = 0.18) had an influence on cancer-unrelated death risk. No statistically significant impact of the side of surgery or histological type of tumor on death risk was found in this analysis.
In the univariate model for the group without PPE, the overall death risk was increased by older age (HR = 1.038, 95% CI: 1–1.07, p = 0.03), higher N stage (HR = 1.58, 95% CI: 1.23–-2.04, p < 0.001) and more advanced clinical staging (HR = 1.36, 95% CI: 1.01–1.85, p = 0.04). The lower value of the T descriptor in this group influenced increased death risk with the result on the threshold of statistical significance (HR = 0.62, 95% CI: 0.38–1, p = 0.052). Progression of the N stage (HR = 1.98, 95% CI: 1.36–2.89, p < 0.001) and more advanced clinical staging (HR = 1.94, 95% CI: 1.24–3.04, p = 0.003) increased cancer-related death risk. The multivariate analysis in the group without PPE revealed that older age (HR = 1.04, 95% CI: 1.01–1.08, p = 0.009), a lower value of the T descriptor (95% CI: 0.4–0.99, p = 0.04) and progression of the N stage (HR = 0.63, 95% CI: 1.26–2.13, HR = 1.64, p < 0.001) were independent statistically significant factors for increased overall death risk. An independent statistically significant cancer-related death risk factor was progression of the higher N stage (HR = 1.97, 95% CI: 1.34–2.91, p < 0.001). A lower value of the T descriptor did not have a significant influence in this group of patients (95% CI: 0.26–1.12, HR = 0.54, p = 0.09). The multivariate model for the group without PPE, which included sex, age, and descriptors T and N as covariates did not reveal any statistical significance for cancer-unrelated deaths. In the univariate model based on the two groups combined, recovering from successfully treated PPE was a statistically significant factor decreasing overall death risk (HR = 0.38, 95% CI: 0.22–0.66, p < 0.001), as well as cancer-related death risk (HR = 0.29, 95% CI: 0.11–0.75, p = 0.01). Older age was a statistically significant factor increasing overall death risk (HR = 1.04, 95% CI: 1.01–1.07, p = 0.009), as well as cancer-unrelated death risk (95% CI: 1.01–1.12, HR = 1.06, p = 0.01). More advanced clinical staging was a statistically significant factor increasing overall death risk (HR = 1.37, 95% CI: 1.03–1.82, p = 0.02) and cancer-related death risk (HR = 1.95, 95% CI: 1.27–3.01, p = 0.002). Similarly, progression of the N stage statistically significantly influenced the former (HR = 1.48, 95% CI: 1.17–1.86, p < 0.001) and the latter (HR = 2.01, 95% CI: 1.4–2.85, p < 0.001). In the univariate model based on the two groups combined, a reduced death risk in the PPE group was noted (Table IV). In the multivariate model including PPE, T and N status, age and sex in both groups combined, recovery from PPE was a statistically significant factor reducing overall death risk (HR = 0.32, 95% CI: 0.18–0.58, p < 0.001) as well as cancer-related death risk (HR = 0.21, 95% CI: 0.0–0.6, p = 0.003). A similar influence was found in the progression of the N stage on the former (HR = 1.49, 95% CI: 1.18–1.88, p < 0.001) and the latter (HR = 1.93, 95% CI: 1.34–2.76, p < 0.001). Older age was an independent statistically significant factor increasing overall death risk (HR = 1.05, 95% CI: 1.02–1.08, p = 0.002) and cancer-unrelated death risk (HR = 1.07, 95% CI: 1.02–1.13, p = 0.009). PPE, sex, and descriptors T and N did not significantly influence cancer-unrelated death risk in this particular model.
Discussion
The introduction of the AT method in clinical practice was the driving force for the examination of the relationship between the effects of PPE on overall survival after pneumonectomy [16]. Treatment outcomes achieved thanks to this method turned out to be highly efficacious and safe in implementation. One aspect of this analysis was to draw attention to the positive impact of recovering from PPE on survival in patients who had been selected for pneumonectomy due to NSCLC. As the number of pneumonectomies performed worldwide has declined (14 pneumonectomies in 2020 in the authors’ center), it is reasonable to infer that the chosen period is best suited for making comparisons due to the largest possible database, drawing on homogeneous criteria for stage assessment [20, 21]. However, it should be noted that pneumonectomy is performed when parenchyma preserving surgery is not possible. This tendency was clearly presented in a study by Chang et al., who found that amongst 20,131 lung cancer patients undergoing surgical treatment, only 171 (0.85%) had pneumonectomy [21].
The two groups did not differ statistically significantly in terms of proportions of the variables examined. The impact of adjuvant treatment was of comparative nature, as it was used in approximately 60% (59.37% vs. 63.54%) of patients treated surgically in both groups (Table I). Due to a lack of comprehensive data on the G descriptor (grading) of tumor, CAE level, and EGFR, KRAS, ALK, ROS1, and PDL-1 mutation, genes were not subject to comparison. Because of their practical contribution to the management of the disease, future research will require such assessment. This juxtaposition of results indicated the superiority of survival outcomes in the PPE group over the group with uneventful recoveries (Table III). A statistically significantly improved outcome was observed in the comparison of overall deaths (p < 0.001) as well as cancer-related deaths (p = 0.003) (Figures 1, 2). According to Cox’s analysis recovering from PPE reduced overall death risk by approximately 2.5-fold (p < 0.001), cancer-related death risk by approximately 3.5-fold (p = 0.01), and cancer-unrelated death risk by 1.2-fold (p = 0.66), although the latter parameter was not statistically significant (Table IV). Individual factors and their impact were subject to assessment in both study groups. The influence of the T value on the results obtained was equivocal. The univariate analysis for the PPE group revealed that the increase of the T value increased overall death risk (p = 0.02) and cancer-unrelated death risk (p = 0.04). Increase of the T value also increased overall death risk in the multivariate analysis for the PPE group (p = 0.03). On the other hand, there was no statistically significant association between the T value and cancer-related deaths in the univariate analysis (p = 0.31) and only borderline statistical significance (p = 0.054) for cancer-related deaths in the multivariate analysis in the PPE group. This phenomenon might be due to the small size of the PPE group, few cancer-related deaths (5/32 – 15.63%), as well as other impactful factors (e.g., recovering from PPE). Paradoxically, the tendency turned out to be reverse in the group with uneventful recoveries. Lower T values increased the risk of death. This trend was observed in overall deaths (p = 0.04) in the multivariate analysis, and it remained on the threshold of statistical significance (p = 0.052) in overall deaths in the univariate analysis. This situation may result from the downplayed relationship between the T value and clinical staging of the tumor. It is implied by the 7th edition of TNM, where changes in the clinical staging of the tumor were based mainly on updating T and M values [22]. Such a situation regarding big tumors T2 (> 7 cm) and T3, with the N0 stage in radical resection was described by Wrona and Jassem, demonstrating improved survival in patients with a T3 tumor as opposed to patients with a T2 tumor [23]. Increase of the N value turned out to be a statistically significant death risk factor in the univariate analysis (overall deaths – p < 0.001 and cancer-related deaths– p < 0.001) and in the multivariate analysis (overall deaths – p < 0.001 and cancer-related deaths – p < 0.001) in the group without PPE as well as in both groups combined (overall deaths – p < 0.001 and cancer-related deaths in the univariate analysis – p < 0.001, overall deaths – p < 0.001 and cancer-related deaths in the multivariate analysis – p < 0.001). Such dependence was not found in the group with PPE, which may also suggest its beneficial properties for patients. The above conclusion is further confirmed by clinical staging analysis. Increased death risk due to progression of the disease was shown in the univariate analysis for the group without PPE (overall deaths – p = 0.04, cancer-related deaths – p = 0.003) in both groups combined (overall deaths – p = 0.02, cancer-related deaths – p = 0.002); however, it did not have a significant influence in the PPE group (p = 0.57 – overall deaths; p = 0.51 – cancer-related deaths; p = 0.81 – cancer-unrelated deaths). In the univariate analysis older age increased cancer-unrelated death risk (p = 0.04) in the PPE group, and overall death risk in the group without PPE (p = 0.03). In both groups combined older age increased overall death risk (univariate analysis – p = 0.009, multivariate analysis – p = 0.002) and cancer-unrelated death risk (univariate analysis – p = 0.01, multivariate analysis – p = 0.009). This conclusion is uncontroversial. The most important premise of the univariate and multivariate Cox analysis is a statistically significant reduction of overall death risk as well as cancer-related deaths in the group where patients have recovered from PPE. These results converge with comparative results obtained from a study of two groups of 31 patients from our previous analysis of overall deaths in both groups combined [4]. Predicted 5- and 10-year survival rates (71% and 59%, respectively) in the study of 32 patients reveal increased overall survival as opposed to the data presented by Mountain and Dresler, who reported outcomes of surgical management of lung cancer without PPE, assessed by means of the 6th edition of TNM [24]. They also show improvement compared to subsequent publications [5, 25]. The obtained excellent survival rates in clinical stage IIB, IIIA and IIIB (93.75% of total cases) suggest that PPE has a positive influence on overall survival in this group of patients. The additional conditions accompanying PPE seem to be important, such as: initial surgical radicality, development of PPE with the involvement of the largest possible area for such a process, common place of occurrence of cancer and empyema, and finally the durability of empyema and potential immunization for a period of months, and even years. The destructive effect of povidone-iodine on the cancer cell wall may be significant as well. Further hypotheses may be related to, among other factors, improvement of activity of fibroblasts and antigen-presenting cells (APC), lymphocyte function and the activity of the CTLA-4 gene as well as the PD-1/PDL-1 system, which requires further research.
Conclusions
Pleural empyema in patients who have undergone radical pneumonectomy for NSCLC prolongs survival compared to patients with uneventful recoveries. Increased survival in the group of patients who have undergone surgical treatment for lung cancer and developed postoperative pleural empyema requires further research on the mechanism of this phenomenon so that this knowledge can be used in future practice.






