Introduction
Androgenetic alopecia (AGA) is the most common form of hair loss in men. Its causes are multifactorial, but the inhibition of the Wnt/β-catenin pathway by Dickkopf-related protein 1 (DKK1) and dihydrotestosterone (DHT) are cited as the most important factors [1–9]. The Wnt/β-catenin pathway regulates many physiological processes, including proliferation, differentiation, apoptosis, and maintenance of cell homeostasis. Wnt proteins, Wnt3a and Wnt10b, are crucial for maintaining thinning hair or regenerating lost hair. The DKK1 protein acts as an inhibitor of the Wnt protein. DHT is primarily formed from testosterone via 5a-reductase, but an alternative pathway derived from cholesterol metabolism is also possible [1, 4, 10]. Receptor sensitivity to DHT is an inherited trait. Those with higher sensitivity have been observed to have earlier symptoms [4]. DHT indirectly inhibits β-catenin through the phosphorylation of glycogen synthase kinase 3β (GSK-3β), leading to its subsequent degradation in proteasomes (Figure 1) [4, 5, 9].
Figure 1
Wnt/β-catenin signalling pathway and androgen metabolism in the aetiology of androgenetic alopecia
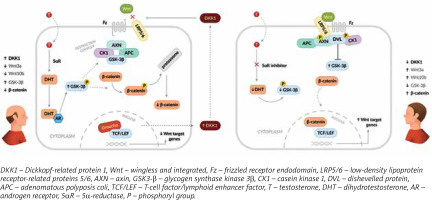
The AGA-inducing processes result in a shortened anagen phase, degradation of microvessels, and gradual miniaturisation of follicles until complete atrophy in the androgen-dependent zone, replaced by extracellular matrix proteins [7, 11, 12]. Fibrosis observed in the middle and late stages of miniaturisation reduces the efficacy of both systemic and topical treatments for AGA. Prevalence correlates with age and race. AGA is more commonly reported in Caucasians, and its incidence increases with age. It affects at least 50% of men under 50 years and 70% of men over 70 years [7, 11, 12]. The Hamilton-Norwood scale can be used to determine the clinical severity of alopecia. In men, the lesions typically occur on the vertex and frontotemporal region [5]. For male patients, the diagnosis can usually be made based on their medical history and clinical symptoms. However, a trichoscopic examination is recommended (Table 1). It can demonstrate the effectiveness of a specific therapy and encourage the patient to adhere to the recommended treatment [1, 13–15].
Table 1
Trichoscopic findings in androgenetic alopecia
| Hair shaft thickness heterogeneity |
| Vellus hairs |
| Pilosebaceous units with only one hair |
| Yellow dots |
| Hair shaft thinning |
| Perifollicular hyperpigmentation |
| Wavy hair |
| Honeycomb pigment pattern |
The condition of scalp hair can have a significant impact on an individual’s self-perception, attractiveness, and overall quality of life. Certain pathologies may also increase the risk of developing diseases, such as depressive episodes [2, 16–19]. For treatment, the most commonly used options are topical minoxidil at 2% and 5%, or oral finasteride, which have been approved by the US Food and Drug Administration. However, there is a wide range of primary and supportive treatments available [11, 12, 20]. It is important to note that successful treatment should be continued for as long as possible, as discontinuing treatment will result in recurrence of the condition. In-office methods for hair restoration include microneedling (MN) and the use of platelet-rich plasma (PRP) (Figure 2). MN is a minimally invasive procedure that involves puncturing the skin surface, specifically the scalp, to produce localised redness and pinpoint bleeding. This method is fast, inexpensive and associated with few side effects [5, 8, 10, 21–26]. The most common devices used for the procedure are derma-rollers or pens. Due to the reduced tissue trauma, the disposability of pen needles, and the ability to adjust the depth of penetration, pens appear to be the dominant device [6, 21, 22, 27]. Microdamage to the skin stimulates a regenerative cascade with the release of growth factors such as platelet-derived growth factor, transforming growth factor α and β, connective tissue activating protein, connective tissue growth factor and fibroblast growth factor, leading to neovascularisation [2, 9, 10, 28]. PRP has found wide application in many medical fields, such as orthopaedics and traumatology, maxillofacial surgery and dentistry. Since 2006, the first described attempts have been made to evaluate the efficacy of PRP in the treatment of androgenetic alopecia. The induced regenerative cascade is explained by the action of platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor, insulin-like growth factor-1 [2, 28].
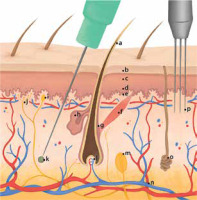
Figure 2
Skin cross-section with a presentation of penetration depth during microneedling and platelet-rich plasma administration: a – hair shaft, b – stratum corneum, c – stratum granulosum, d – stratum spinosum, e – stratum basale, f – musculus arrector pili, g – hair follicle bulge, h – sebaceous gland, i – Tactile corpuscles (Meissner’s corpuscles), j – free nerve endings, k – platelet-rich plasma deposition, l – papilla, m – lamellar corpuscle (Vater-Pacini corpuscle), n – subcutaneous blood vessels, o – sudoriparous glands, p – needles used during microneedling
Aim
The objective of this study is to assess the effectiveness of microneedling monotherapy (MN), combination of microneedling with topical 5% minoxidil (MN + MNX) and autologous platelet-rich plasma (PRP) in men with androgenetic alopecia.
Material and methods
The research was carried out on a specific group of men aged between 20 and 50 years, who had lesions ranging from II to VI on the Hamilton-Norwood scale. The subjects were randomly assigned to one of three groups: group A (MN), group B (MN + MNX), and group C (PRP), with 31, 31, and 30 patients, respectively. The study consisted of three treatments, each 1 month apart, and a final evaluation of the effects 2 months after the third treatment. All eligible patients completed the study. At baseline, the depth of microneedling was set at 1.0 mm. The treatment continued until local redness and pinpoint bleeding of the scalp were achieved. In addition, group B was instructed to apply 2 ml of 5% MNX daily, but not within 24 h after the microneedling procedure. For group C, whole venous blood was collected into anticoagulant tubes. The material for the procedure was obtained using the single-spin technique at 1100G for 10 min. After centrifugation, about two-thirds of the supernatant, which contained platelet-poor plasma, was removed. The remaining PRP was then injected between the hair follicles using mesotherapy needles (30G, 4 mm). A comparison was made between the efficacy of these methods at the 5-month follow-up by two independent investigators. The methods used included physical examination, hair density analysis, hair thickness analysis, macroscopic photographic assessment, obtaining information on the patient’s age of onset of hair loss and age of onset of hair loss in the patient’s immediate family. Exclusion criteria for the study were: use of other topical medications on the scalp (rubs, therapeutic shampoos), coagulation disorders, anticoagulant therapy, active liver dysfunction associated with abnormal function tests, any oncological disease within the last 5 years, acute hepatitis A, B, C, D, E or chronic hepatitis B, C, D, E, HIV infection, alcohol dependence, dependence on opiates, cannabinoids, sleeping and sedative drugs, cocaine, hallucinogens, volatile organic solvents and other psychoactive agents, any mental illness, scalp lesions that, in the individual physician’s opinion, constitute a contraindication to injection or microneedling, lack of patient cooperation.
Statistical analysis
Data are presented as mean ± standard deviation, maximum, minimum and median with 1st and 3rd quartiles for quantitative variables and as number of cases with a percentage for qualitative and semi-quantitative variables. In addition, a 95% confidence interval (95% CI) was used for differences in values of variables describing hair parameters between the beginning and the end of the study. For qualitative variables, Pearson’s χ2 test was used for multivariate table analysis and Fisher’s exact test for quadratic tables. Quantitative variables were assessed using Student’s t-test for two group comparisons and repeated measures analysis of variance. Normal distribution was assessed using Q-Q plots and histograms, with the Levene test used to assess homogeneity of variance. The assumption of homogeneity of variance was tested with the Mauchly’s test; if it was not met, the Greenhouse-Geisser correction was applied. The analysis was performed using the R language in the RStudio environment. P-values of less than 0.05 were considered significant.
Results
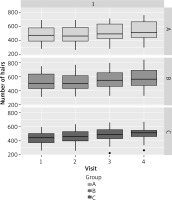
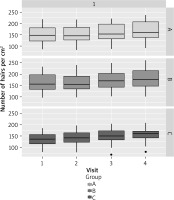
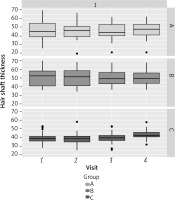
Patients in group C experienced hair loss significantly earlier than those in groups A and B, and were younger at the time of study participation. The age of paternal hair loss was similar in all groups (Table 2). The results obtained in each group and at each visit in terms of hair number, hair density and hair shaft thickness are presented in Tables 3–5 and Figures 3–6. Patients in group A showed an average increase of 53 hairs over 4 visits. In patient group B, there was an increase of 55 hairs during follow-up, while patient group C experienced an increase of 68 hairs (Table 6). The average hair density increased by 17 units over the four visits in patient groups A and B. In contrast, patient group C experienced an increase of 21 units in the number of hairs. The hair thickness mean increased by 0.1 µm over four visits in patient groups A and B. The largest increase in hair thickness was observed in patient group C, where it was 4 µm. Throughout the study, each group showed a significant increase in hair growth, with no significant differences between the groups. An increase in hair growth was observed in each group of patients after four visits. The mean change in hair count ranged from 53.6 to 67.5. There was no significant difference between the methods (Table 7). A significant difference was also observed in the density of hairs per cm2 over the course of the study, with no clear advantage for any of the methods (Table 8). The mean change in hair count ranged from 16.8 to 21.1, with no significant difference observed between the methods (Table 9). A significant increase in hair thickness over the course of the study was observed in group C only. In the other groups, the increase in thickness appeared to be almost imperceptible (Table 10). There was a difference of 3.96 in hair shaft thickness within group C, while the other groups showed only minor differences (Table 11).
Table 2
Age of hair loss in a patient and their father
Table 3
Descriptive statistics of the number of hairs in the study group
Table 4
Descriptive statistics of hair density in the study group
Table 5
Descriptive statistics of hair shaft thickness in the study group
Table 6
Analysis of variance for change in the number of hairs during the study
| Effect | F | P-value | ges |
|---|---|---|---|
| Group | 3.088 | 0.051 | 0.063 |
| Visit | 133.493 | < 0.001 | 0.041 |
| Group* visit | 2.328 | 0.06 | 0.001 |
Table 7
Difference in hair count between the initial and end points of the study with 95% confidence interval
| Group | Variable | Mean | SD | SE | Low_CI | Up_CI | P-value |
|---|---|---|---|---|---|---|---|
| A | d n hair | 53.6 | 37.6 | 6.75 | 40.4 | 66.9 | 0.299 |
| B | 54.8 | 42.4 | 7.61 | 39.9 | 69.7 | ||
| C | 67.5 | 34.3 | 6.27 | 55.2 | 79.8 |
Table 8
Analysis of variance for change in hair density during the study
| Effect | F | P-value | ges |
|---|---|---|---|
| Group | 3.088 | 0.051 | 0.063 |
| Visit | 133.493 | < 0.001 | 0.041 |
| Group* visit | 2.328 | 0.06 | 0.001 |
Table 9
Difference in hair density between the initial and end points of the study with 95% confidence interval
| Group | Variable | Mean | SD | SE | Low_CI | Up_CI | P-value |
|---|---|---|---|---|---|---|---|
| A | d n hair cm2 | 16.8 | 11.8 | 2.11 | 12.6 | 20.9 | 0.299 |
| B | 17.1 | 13.3 | 2.38 | 12.5 | 21.8 | ||
| C | 21.1 | 10.7 | 1.96 | 17.3 | 25 |
Table 10
Analysis of variance for mean hair thickness during the study
| Effect | F | P-value | ges |
|---|---|---|---|
| Group | 11.183 | < 0.001 | 0.19 |
| Visit | 16.413 | < 0.001 | 0.012 |
| Group: visit | 5.056 | < 0.001 | 0.007 |
Table 11
Difference in hair shaft thickness between the beginning and the end of the study with a 95% confidence interval
| Group | Variable | Mean | SD | SE | Low_CI | Up_CI | P-value |
|---|---|---|---|---|---|---|---|
| A | d mean thickness | 0.07 | 5.92 | 1.06 | –2.02 | 2.15 | 0.002 |
| B | 0.16 | 4.58 | 0.82 | –1.45 | 1.77 | ||
| C | 3.96 | 3.63 | 0.66 | 2.66 | 5.25 |
Discussion
The best macroscopic results were recorded in patients under 30 years of age and those who first observed signs of androgenetic alopecia about 5 years ago (Figures 7 and 8). No patients reported any deterioration of the scalp during the 5-month follow-up. In choosing the parameters of the therapeutic algorithm, we were guided by the following criteria: comfort, best possible ratio of potential results to price. In the case of MN, the range of algorithms presented in the literature is very wide. The number of treatments in the algorithms ranges from 3 to 52, intervals from 1 week to 1 month, and penetration depths from 0.25 mm to 2.5 mm [3, 6, 7, 23–25, 29–34]. The choice of 3 treatments at 1-month intervals was guided by the choice of parameters to an effective minimum. The depth of penetration was set at 1.0 mm because of greater regularity of the length of the microchannels formed and the lower risk of damaging hair follicle bulge located 1.0–1.8 mm below the scalp surface [3, 21, 27]. We assumed that the depth could be reduced if subjective pain was severe, but the procedure was still performed to achieve redness and pinpoint bleeding.
Figure 7
Patient aged 24 years (group A), first symptoms of alopecia at the age of 20 years. No comorbidities. He did not use any hair loss prevention methods in the past. A – baseline, B – after 5 months
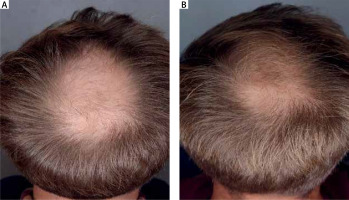
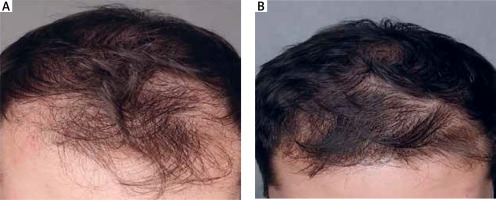
Figure 8
Patient aged 25 years (group B), first symptoms of alopecia at the age 23 years. No comorbidities. In the past he used 5% topical minoxidil for 1 month with no effect. A – baseline, B – after 5 months
In the case of PRP, there is an equally wide divergence in the therapeutic algorithms used. These range from the centrifuge (horizontal or angular), the centrifugation method (single or double spin), the use of an activator or separating gel in the tube, to overload and centrifugation time [28, 29, 33, 35–39]. The choice of 1100 G for 10 min using the single spin method was mainly guided by the need to minimise the risk of platelet damage, which plays an important role in the PRP method, and to reduce the risk of pyrogenicity of the procedure.
Although the treatment algorithms were kept to a reasonable minimum, satisfactory results were achieved in the study groups at the 5-month follow-up. The lack of statistically significant differences between the groups in hair volume and density may be explained by: too short follow-up period, lack of patient’s discipline in applying MNX at home, manual measurements and analysis error. Factors that should be considered in the design of future studies include: a 12-month follow-up period, analysis of different penetration depths and treatment intervals for effects in MN, use of a more convenient form of drug delivery (e.g. oral minoxidil), use of different centrifugation algorithms in PRP, introduction of a placebo group (e.g. performing MN treatment without an attached cartridge or with a roller without needles, administration of 0.9% NaCl instead of PRP), use of an AI-assisted device in the evaluation of microscopic images. The results obtained should be taken into account when proposing individual therapies to patients. They vary considerably in terms of pain during the procedure, the cost of a single procedure, and the need for regular external application of the drug, or the lack thereof. In each case, the therapeutic management algorithm should be discussed with the patient, taking into account their preferences, motivations and financial capabilities. The methods presented appear to be a promising therapeutic alternative for patients for whom a treatment with standard agents is not possible or recommended, for various reasons.