Introduction
Total mesorectal excision (TME) is currently the standard surgical approach in the treatment of rectal cancer [1, 2]. Despite progress in the technique, surgery for low rectal cancer remains technically challenging. Moreover, according to several well-designed randomized controlled trials, introduction of minimally invasive techniques, although beneficial in terms of short-term outcomes, did not lead to significantly improved oncological outcomes [1, 3, 4]. Improvement of the results can be expected only by raising the quality of the surgical technique of mesorectal excision. The final result can be easily assessed by pathological evaluation of the resected specimen [5].
To overcome intraoperative difficulties of TME performed by the transabdominal approach (both open and laparoscopic), transanal total mesorectal excision (TaTME) has been proposed [6]. This promising technique improves visualization and dissection of the mesorectal fascia plane, especially in obese patients or in a narrow, irradiated pelvis. It is expected that this will result in more precise and atraumatic dissection, improving quality of the operative specimen and reducing positive resection margins [6–9].
It has been shown that the annual volume of the surgical centre and the team’s experience are crucial to achieve good oncological outcomes in rectal cancer treatment [10, 11]. To achieve a high level of proficiency, surgeons must go through the learning curve that is estimated to require at least 50 cases of laparoscopic low anterior resection [12, 13]. Since TaTME is a relatively new technique, the number of operations that should be performed to reach a stable level of skill is still poorly defined. Thus, we designed a study to evaluate the learning curve of TaTME based on our experience.
Aim
The aim of the study was to evaluate the learning curve of TaTME based on a single centre’s experience.
Material and methods
Study design
We performed a retrospective analysis of a prospectively collected database. Consecutive patients operated on with TaTME technique were included in the study. We are a tertiary referral department in a university hospital with an annual volume of at least 50 rectal resections. Transanal total mesorectal excision was introduced in 2014 as a procedure for low rectal cancer (< 5 cm from the anal verge). Our department is a participant in the COLOR III trial [14]. In this study we analysed a single surgeon’s experience. In all presented cases the procedures were performed by the same experienced surgeon.
Every patient underwent diagnostic colonoscopy with tumour biopsy and subsequent pathological confirmation of adenocarcinoma. All patients had pelvic magnetic resonance imaging (MRI) and thoracic and abdominal computed tomography (CT) scan evaluation as a routine preoperative workup. Patients classified as stage T3 or N+ in MRI were submitted to neoadjuvant treatment. In case of suspicion of threatened circumferential resection margin (CRM) or infiltration of the internal anal sphincter, MRI was repeated after completion of chemoradiotherapy.
Before implementation of TaTME, the surgical team underwent training in reference centres that included cadaver-based hands-on training. The operative technique is described elsewhere [6]. As a modification of the original technique, we use the TEO TEM platform by Karl Storz. The procedure is routinely performed in a one-team approach. The anastomosis is performed with a circular stapler or alternatively hand-sewn if stapled anastomosis is technically not feasible. The enhanced recovery after surgery (ERAS) protocol with good compliance was used in perioperative care in all cases [15–17].
The surgical specimen was assessed by an experienced pathologist. The quality of mesorectal excision was evaluated in accordance with Quirke criteria [18].
For the purpose of the study we performed CUSUM analysis of postoperative morbidity, intraoperative adverse effects, operative time and quality of resected specimen. Based on CUSUM curves we divided the patients into two groups: group 1 consisted of patients operated on during the learning curve and group 2 patients operated on after reaching the plateau of the learning curve.
Statistical analysis
All data were analysed with StatSoft Statistica version 13.0 PL (StatSoft Inc., Tulsa, OK, USA). The results are presented as mean ± standard deviation (SD), median and interquartile range, and odds ratios (ORs) with 95% confidence intervals (CIs) when appropriate. To assess statistical significance of qualitative data differences between subgroups, Pearson’s χ2 test and discriminant analysis were used. CUSUM curve analyses were used to estimate cut-off values in terms of the number of procedures performed to reach stabilisation of the learning curve. Results were considered statistically significant when the p-value was less than 0.05.
Results
Patients
We included 66 patients who underwent TaTME performed by the same surgeon. Forty-four (66.7%) patients were male. Median age was 64 (56–71) years. Fifty-four (81.82%) patients required neoadjuvant treatment. Eighteen (28.5%) patients had stage 0 according to the AJCC classification, 16 (23.8%) stage I, 14 (17.5%) stage II, 16 (4.8%) stage III and 2 (3.2%) stage IV. The characteristics of groups are presented in Table I.
Table I
Characteristics of study groups
Analysis of the CUSUM charts
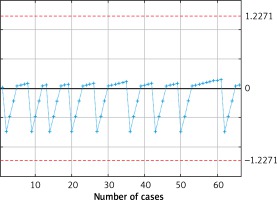
We observed a drop in the postoperative morbidity rate after the 30th case and in intraoperative adverse effects after the 35th case (Figures 1 and 2). We also observed stabilization in the operating time at the 40th case (Figure 3). After that, we observed a slight descent of the curve. We did not identify changes in the learning curve regarding pathological quality of the specimen (Figure 4). Based on those analyses, we estimated that at least 40 surgery cases are needed to stabilize the learning curve. Therefore, we divided our patients into two groups: group 1 (first 40 cases) and group 2 (remaining patients).
Sub-group analysis
Median overall operative time was 240 (IQR: 210–280) min. In total, 6 intraoperative adverse events were noted (4 purse string failures, 1 inability to maintain stable insufflation of the working space in the pelvis and 1 case of bleeding in the perineal step of the procedure). There were no significant differences between the groups (p = 0.216).
Postoperative complications occurred in 22.73% of patients (Table II). There were significant differences between the groups (p = 0.019) – a majority of complications occurred within first 30 cases (12/15, 80%).
Table II
Number of complications
Pathological outcomes were stable during the learning process (Table III). One patient in group 1 had positive CRM and 1 patient in group 2 had positive distal resection margin (DRM). Median CRM and DRM did not differ between the groups (p = 0.542 and p = 0.573, respectively). There were no differences regarding pathological quality of the specimen.
Table III
Pathological outcomes
We observed a significant difference in the readmission rate (p = 0.041). Most of the readmissions occurred in group 1 (63%). The median overall hospital length of stay (LOS) was 5 days and was not statistically different between the groups (p = 0.375). However, median LOS in group 1 was longer by one day (6 (4–12)).
Discussion
This is one of the first studies evaluating the learning curve of TaTME. We found that a significant drop of perioperative complications and operative time happens after the first 40 cases.
Available data about TaTME are sparse and come from large, high-volume centres, which specialize in laparoscopic surgery [19, 20]. Moreover, the definition of TaTME is not unified and there are several approaches that differ substantially yet still are called TaTME. The St. Gallen consensus on TaTME implementation delivered a standard technique, which is included as part of the COLOR III protocol [14, 21]. Nonetheless, available studies are heterogeneous regarding both operative technique and patient population. This creates a bias as in the case of the meta-analysis by Hu et al. that included studies assessing different transanal approaches that do not necessarily follow the principles of TaTME [22]. Moreover, almost all currently available studies comparing TaTME to other techniques present data from the middle of the learning curve [23, 24].
Transanal total mesorectal excision is a complex endoscopic procedure that requires a learning curve to obtain stable results. There are several factors that can shorten the learning process, including experience in laparoscopic technique or participation in hands-on cadaver-based courses [21]. It is also a possible explanation of the shorter learning curve in our institution than reported before [25, 26].
Perhaps the most clinically significant parameters taken into consideration when analysing the learning curve are postoperative complications. We observed a drop and stabilisation of the learning curve regarding postoperative complications after the 30th case. Available studies show comparable results of TaTME to laparoscopic TME (LaTME) [9, 27, 28]. However, most of these studies are based on small data samples from the initial period of the TaTME learning curve, whereas LaTME cases are usually matched from larger databases [27, 28]. In large datasets overall morbidity of LaTME is estimated to be around 36% [1]. Lacy et al. reported overall morbidity of 24% with major complications (Clavien-Dindo III–V) reaching the 10% rate after 140 TaTME cases [29]. Moreover, Koedam reported a rate of 17.5% for overall complications after the first 40 cases, which was defined as a cut-off for the learning curve. This trend was not observed by Lee et al., who reported a constant rate of postoperative complications in the range between 42% and 45%. We observed a significant drop in overall morbidity, which allowed us to define a period after learning curve stabilization. During the first period we used hand-sewn or stapled anastomosis, depending on its distance from the anal verge. However, further experiences allowed us to use stapled anastomosis in almost every case, which resulted in a drop of the anastomotic leakage rate.
One of the main problems that might occur while learning TaTME technique is specific intraoperative adverse effects (IAE), which – due to differences in the technique – do not occur in LaTME. In our material, the most common IAE was purse string failure. In theory, this may result in bowel content spillage and contamination of the operative field, including with cancer cells [30]. Other authors also point out that perforation of the bowel wall or gas embolism may occur [19, 31]. Mege et al. revealed that IAE in TaTME are more common than in LaTME, and may also present as urethral injury, bladder perforation or vagina wall damage [27]. A narrow pelvis and difficult anatomy after radiotherapy may also lead to iliac vessel injury [26]. Again, in our material the majority of IAE occurred within the first 35 cases. Lee et al. observed a similar drop from 12% to 6%, although the number of cases required for passing the learning curve was estimated at 51 TaTME procedures [26].
Operative time is probably the most frequently used parameter for evaluation of the learning curve, although it plays a limited role in clinical evaluation. CUSUM analysis revealed its stabilisation after 40 cases from a median of 270 to 210 min. Other authors report an even more significant drop, but it is frequently associated with implementation of a two-team approach [29]. Koedam et al. observed a substantial reduction in the operative time after introduction of the two-team approach. However, both in one- and two-team approaches the operating time did not change significantly with surgical experience [25]. Lee et al. observed shortening of the operative time after the 36th case, although they did not indicate the moment of switching the technique to the two-team approach [26].
Our study has some considerable limitations. Firstly, the study group consists of 66 cases, which limits the power of statistical tests. However, we observed stabilisation of operative parameters after the 40th case, so our material could be considered sufficient. We also evaluated the learning curve of a single surgeon, who is already an expert in minimally invasive techniques. More cases are required to evaluate the learning process in the entire department. In this study we also did not analyse functional and long-term outcomes, which are crucial for patients. Nonetheless, TaTME is still under initial evaluation and none of the studies published in the literature assessed long-term outcomes. We expect such studies in the next few years, as TaTME was introduced in 2010 and long-term observations should become available shortly [6]. They are also one of the crucial points of COLOR III – a randomized multi-centre study comparing TaTME with LaTME [14].
Conclusions
Transanal total mesorectal excision is a promising procedure, but the technique is technically demanding and requires at least 40 cases to finish the learning curve. Having said that, more data are needed to evaluate TaTME and introduce it as a standard procedure for low rectal cancer treatment.