INTRODUCTION
Biological drugs are a new and increasingly important part of modern therapy for many diseases, but they also pose new challenges in terms of long-term safety. One of these are drug-induced hypersensitivity reactions [1]. In this report, we describe a patient with a delayed cutaneous hypersensitivity reaction to evolocumab, a human monoclonal antibody directed against the serine protease proprotein convertase subtilisin/kexin type 9 (PCSK9) used to reduce low-density lipoprotein concentrations in the treatment of familial hypercholesterolaemia (FH) [2, 3].
CASE REPORT
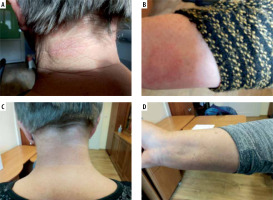
A 67-year-old Caucasian woman was referred to our department because of erythematous exfoliative lesions on the skin of the neck, forearms (Figures 1 A, B) and face. The lesions first appeared after the third administration of evolocumab, given subcutaneously every 2 weeks at a dose of 140 mg for FH. After each of the next three consecutive doses, the lesions worsened for 24 h, persisted for approximately 7 days, and then went into partial remission. They were accompanied by local pruritus and mild symptoms of conjunctivitis and stomatitis, but there were no other alarming signs such as vesicles, bullae or Nikolsky’s sign. Discontinuation of evolocumab resulted in a complete remission (Figures 1 C, D). The patient denied any history of allergic reactions or drug-induced hypersensitivity reactions. Routine laboratory tests (liver transaminases, creatinine, C-reactive protein, peripheral blood counts) showed no significant abnormalities. On skin biopsy of the lesion, haematoxylin and eosin staining showed a perivascular inflammatory lymphocyte infiltration, which may correspond to drug-induced lesions. According to the Naranjo algorithm, the probability scale for an adverse drug reaction was 5 points, indicating that a causal relationship is likely (reaction occurred after a recognised time sequence after taking the drug and was confirmed by withdrawal) [4]. Other in vitro or in vivo diagnostic tests with the drug were not performed because of the high likelihood of a causal relationship between the administration of the drug and the observed symptoms and unavailability of the drug for testing. Symptomatic treatment with bilastine at a dose of 20 mg daily, accompanied by topical treatment with methylprednisolone (ointment 0.1%, od) for the 7 days followed by tacrolimus (ointment 0.03%, bid) for the next 5 days, was performed with good results. Referring to the current recommendation for the classification of cutaneous manifestations of drug hypersensitivity, the observed reaction shared some clinical features of FDE (erythematous plaques, recurrence at the same sites after re-administration), DRESS (eczema-like lesions, localisation on the face, upper trunk, extremities) and MPE (lack of systemic involvement) [5]. It should be taken into account that more than one type of reactions can occur in the same patient [6].
DISCUSSION
The most commonly reported adverse reactions associated with the use of evolocumab are nasopharyngitis, upper respiratory tract infection and back pain [2]. To date, only two cases of drug-induced skin reactions with a temporal relationship to the use of evolocumab have been described in detail [7, 8]. The first, reported by Japanese authors, is the case of a patient who developed an “atopic dermatitis-like rash” after a dose change from 140 mg of evolocumab every 2 weeks to 420 mg monthly after 16 months of treatment. The authors suggest that evolocumab unmasked the patient’s predisposition to atopic diathesis. The skin lesions resolved after symptomatic treatment and did not recur despite continued treatment with evolocumab [7]. In the second case, a maculopapular rash occurred already after the second dose of evolocumab and the drug was discontinued [8]. Compared to the above cases, the causal relationship between evolocumab administration and symptoms in our patient was very convincing as subsequent doses of the drug exacerbated the symptoms, as it happens in controlled drug provocation tests, which are considered the gold diagnostic standard, and furthermore, discontinuation of the drug resulted in a complete remission. There were also no alternative causes for the symptoms. However, it should be emphasized that the complete diagnostic process usually involves skin prick tests and/or intradermal tests with a suspected drug, as well as in vitro assays such as lymphocyte proliferation (transformation) test. In our case, it was not possible to perform these procedures due to both the lack of a sufficient amount of the drug for testing and limited access to validated diagnostic assays. It should be noted that the patient did not report any of the typical danger signs associated with drug-induced hypersensitivity reactions, with the exception of conjunctivitis and oral mucosal erosions [9], but she decided to discontinue evolocumab treatment, because she feared an exacerbation of the existing skin lesions during further treatment. She made this decision despite consulting her cardiologist and assessing the benefits and risks of the therapy. However, in the absence of clinical warning signs, a “treat-through” strategy could be considered, similar to that sometimes used for β-lactam-induced delayed reactions [10]. When carrying out any in vivo diagnostic and therapeutic procedures in drug hypersensitivity, the safety conditions that are currently recommended should be followed [11].
CONCLUSIONS
Due to the chronic nature of the underlying disease and its potentially life-threatening cardiovascular complications, optimal management in cases similar to ours should consider the benefit-risk balance of continued evolocumab. Desensitisation has not been described to date, but given the dosing schedule of evolocumab every 2–4 weeks, the chances of success would appear to be low.