Introduction
The liver is one of the most common sites for metastasis because of its rich blood vessels [1]. About 20% of patients with colorectal cancers present with liver metastases at the time of diagnosis, while 20–50% will develop in the later period [2]. Until recently, radiation therapy was not a curative option for liver metastases because of its potential toxicity to the liver rather than due to the liver movability and was often part of palliative approaches [3]. The whole-liver radiotherapy should not exceed the whole liver tolerance dose of 30 Gy. This dose, however, is not sufficient for tumor control, and there has been no proven contribution of whole-liver radiotherapy to overall survival [4]. This led to the need for target-lesion radiotherapy approaches and the start of reports on high-dose hypofractionated therapies for liver lesions [5, 6]. Stereotactic body radiotherapy (SBRT), which can be broadly called “high-precision radiotherapy”, involving the delivery of high doses only to the target in a very short time, remains valid [7]. Available studies suggest that SBRT is an effective and safe option to treat liver metastases [8, 9]. Apart from SBRT, local treatment options for liver metastases include various ablative procedures such as metastasectomy, radiofrequency ablation, transarterial chemoembolization, transarterial radioembolization, and cryoablation. These procedures can also be used to treat palliative symptoms such as pain and pressure [10].
The major problem in intrathoracic and intraabdominal radiotherapy is target motion due to respiration. For the liver, this deviation ranges from five to 50 mm in the craniocaudal direction [11]. The expansion of the target to include respiratory motion will certainly result in a larger tumor volume than necessary. A correction of this magnitude without using an appropriate technique may cause incorrect tumor contouring, incorrect treatment practices including dosimetry changes, and increased side effects due to the entry of more normal tissues to the treatment site [12, 13]. Several radiotherapy approaches are available to provide safer target treatment such as real-time tracking of moving targets, active breathing control, end-expiratory gating, and deep inspiration breath-hold techniques. Each technique has its unique challenges. Among these are the patient’s inability to have a harmonious respiratory rhythm, inability to follow commands, difficulty in adapting to treatment, and long durations. Accordingly, radiotherapy techniques based on respiratory rhythm may not be suitable for every patient. Stereotactic body radiotherapy with abdominal compression (AC) is a method actively used in the treatment of intra-abdominal and intrathoracic lesions with SBRT [14, 15]. In the AC technique, a constant force is applied to the abdomen. The applied force restricts the motion of the diaphragm and reduces the respiration-induced motion of the upper intra-abdominal organs in particular. It has been demonstrated that the motion of the liver is reduced, the target lesion motion is restricted in a three-dimensional way and this is preserved during radiotherapy fractions by using abdominal fluoroscopy and 4DCT in patients undergoing SBRT with the AC technique [16–19].
The present study aimed to retrospectively review the patients on whom we used the AC technique in the treatment of liver metastases with SBRT and report the outcomes.
Materials and methods
Patient characteristics
A retrospective review of the data of 79 patients who had liver metastases, who did not undergo metastasectomy or any other local treatment for the liver that could substitute radiotherapy, and who underwent SBRT with AC between 2012 and 2021 was performed (Table 1).
Table 1
Patient characteristics
Clinical procedure
For patients scheduled for SBRT with AC, treatment planning images were acquired using the Siemens Biograph Positron Emission Tomography Vision system. The treatment planning was performed using the Eclipse Treatment Planning System V10.0 (Fig. 1). The treatment was administered using the Varian Trilogy Rapidarc HDMLC system. The Macromedics Eamis Abdominal Compression system consisting of a vacuum mattress, wingstep, and knee wedge was used for planning and treatment (Fig. 2). Abdominal compression was applied to the maximally tolerable level by the patient. Positron emission tomography-computed tomography (PET-CT) and magnetic resonance imaging (MRI) were the radiological examinations used to contour the tumor volume and localization on CT images acquired during treatment planning.
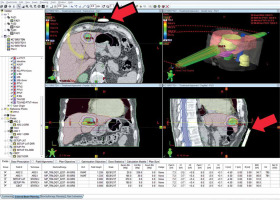
Fig. 1
The figure shows a patient’s treatment plan in the Eclipse Treatment Planning System V10.0. Arrows show the pressure plates of the abdominal compression device
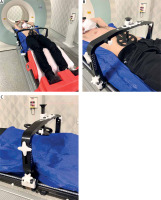
Figs. 2 A, B, C
Photographs showing a patient in the simulation procedure with the all abdominal compression device equipment
There are many modern technical options in liver SBRT applications. Abdominal compression technique was the most appropriate approach for our clinic in terms of cost, infrastructure and the possibilities offered. In the SBRT application with the AC technique, pressure on the abdominal region is applied to restrict the movements of the diaphragm, thus restricting the abdominal breathing and encouraging lung-based superficial breathing. Therefore, approaches such as deep inspiration, force expiration, and breath hold will not be suitable for patients who use AC technique.
The pressure plate part of the device is applied to the soft tissue area between the xiphoid process at the top and the bony triangle formed by the arcus costalis on the right and left, so that it does not touch the bone structures.
Daily image guidance, using onboard CT imaging (4D cone beam-CT), was used to relocalize the target before treatment delivery. During each treatment, the patient’s position was checked by acquiring 4D cone beam-CT scans.
Contrast agent was used in the CT-simulation procedure for RT and in diagnostic MRI scans. Contrast agent was not used in diagnostic PET-CT scans.
A critical dose-volume histogram model was applied to fulfill the constraints for organs at risk (OAR). Liver volume receiving 15 Gy is less than 700 cm3, spinal cord volume of 0.1 cm3 receives less than 18 Gy, volume receiving 15 Gy for both kidneys is less than 35%, volume receiving 21 Gy for duodenum, small bowel, esophagus, and stomach is below 1%, the volume that receives 30 Gy for the heart is below 1%, and the rib volume of 30 cm3 receives less than 30 Gy [20].
The median radiotherapy dose was 30 Gy (20–50 Gy) and the median fraction size was 8 Gy (8–10). The most commonly used dosing regimen is 30 Gy in three fractions. In 25 patients median EQD2 was 97 (50–198), (α/β = 10). Stereotactic body radiotherapy was planned and administered by using dynamic conformal arcs generated by a linear accelerator with energies of 6 to 18 mV. The dose was prescribed to the isodose line that covered the planning target volume (PTV) (80–90% isodose line). The volumes used in the planning were as follows: gross tumor volume (GTV) (cc) 14.8 (1.3–154.7), PTV (cc) 65.8 (16.6–410.8).
No special approach was used for the inclusion of patients in the study. Patients who could be treated with SBRT with the AC technique in liver metastases were treated according to international guidelines and in the order of arrival, and then followed up. After the appropriate follow-up period, the patients were reviewed retrospectively.
Patients were evaluated 1 month after treatment and then every three months for the first two years and then every six months thereafter through clinical physical examination, radiological imaging, and blood tests. The Response Evaluation Criteria in Solid Tumors (RECIST V1.1) were used to assess radiological tumor response. PET-CT examinations were evaluated by a nuclear radiologist and MRI examinations by a radiologist. The term “in-field” was used for the intrahepatic target lesion volume treated with radiotherapy. The treatment response in this volume with PET-CT and MRI examinations performed for post-treatment response assessment was considered in-field local control (ifLC). Recurrence in the intrahepatic volumes that did not receive radiotherapy was expressed using the term out-field local control (ofLC). Adverse events within the first three months were defined as acute toxicities and adverse events after three months were defined as late toxicities. Acute and late toxicities were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. In-field local control and toxicity were considered as primary endpoints of the study.
The general purpose of using radiotherapy equipment is to increase treatment accuracy. However, radiotherapy equipment can complicate setup and increase device occupancy. Knowing these details can be important to determine the clinical relevance of any technique. This also applies to the AC technique. In order to evaluate the compliance with daily practice, criteria such as “time to start the treatment” and “number of repositionings” were determined quantitatively.
Using the AC technique, the time spent by the patient on the device and the number of shots required to reach the correct position were calculated. According to the literature, 10 minutes or more between the cone beam CT scan and the start of treatment in a patient who is treated is expressed as a prolonged time [21]. In our study, the time between cone beam CT scan image acquisition time and the time to start treatment was calculated separately for each patient and for each treatment fraction. It was also grouped as less than 10 minutes and above. This time interval was considered as the time taken to give the correct treatment position to the patient, and the length of the time was considered as a negative factor.
In addition, the number of repeated cone beam CT scans until the start of treatment was calculated for each patient and for each treatment fraction. Each cone beam CT scan repetition was evaluated as repositioning, and the “number of repositionings” was evaluated as a negative factor.
Results
This study examined 79 patients who underwent SBRT with the AC technique between 2012 and 2021. Among those treated, colorectal cancer was the most common type of primary tumor type (n = 32, 40.5%). Other cancer types were breast cancer in 16 (20.3%), lung cancer in seven (8.9%), gastric cancer in five (6.3%), pancreatic cancer in five (6.3%), cholangiocarcinoma in four (5.1%), renal cell carcinoma in 3 (3.8%), bladder cancer in two (2.5%) patients, ovary cancer in one (1.3%), esophageal cancer in one (1.3%), parotid gland cancer in one (1.3%), prostate cancer in one (1.3%), and laryngeal cancer in one (1.3%) patient. The median follow-up of all patients was 14 (4–73) months from the end of treatment to the last follow-up visit or death. After treatment, one-year ifLC was 46.4%, six-month ifLC was 76%, one-year ofLC was 13.6%, and six-month ofLC was 25%.
Considering adverse effects, only eight patients had acute gastrointestinal toxicity according to CTCAE Version 5.0. The gastrointestinal adverse effects were grade two nausea in one patient, grade one nausea in two patients, grade one bloating in three patients, and grade one gastroparesis in two patients. There was no adverse effect on systems except the gastrointestinal system in the acute period and no late toxicity was observed in any patient.
In our study, “time to start the treatment” was calculated as median eight minutes and range 5–16 minutes. This period was similar to the literature findings [22]. The number of patients with a time of ten minutes or more was 23. There were seven patients in the group of patients who were taken for 10 minutes or more, with a maximum intake time of 13 minutes. “Number of repositionings” was found to be median 2.6, range 1–8, and this was in accordance with general radiotherapy patient recruitment principles.
Discussion
Approaches that reduce the dose to normal liver and surrounding organ tissues in radiotherapy enable the amount of therapeutic dose to the tumor to be increased and side effects to be managed. These approaches include defining the tumor volume to be treated with radiotherapy more accurately using examinations such as MRI and PET-CT, increasing the conformity index of the tumor dose in the treatment planning system, and using methods that restrict the tumor motion induced by respiration during the treatment or using tumor tracking methods.
Strategies to reduce respiratory-induced motion of intrathoracic and intra-abdominal tumoral lesions are based on six methods included in the 2008 Guidelines for Radiotherapy Planning [23]: inhalation of oxygen, AC, learning regular respiratory patterns, breath-hold technique, gating with respiration, and real-time tumor-tracking. Details of the techniques are described in the report of the American Association of Physicists in Medicine Task Group 76 [24].
Stereotactic body radiotherapy with AC is useful for reducing respiratory motion in moving tumors and nearby normal tissues and is often used along with abdominal bands, body shells, and stereotactic body frames. Stereotactic body frames typically include vacuum cushions and pressure plates that are pressed against the abdomen. The accuracy and reproducibility of both the body frame and the pressure plate were reported in a comprehensive assessment report [25]. However, the use of body frames is associated with some problems such as set-up uncertainties, displacement of the lesion with increasing respiratory motion in the anteroposterior direction, and physical pain. Pressure plates may not be used in patients with a previous colostomy who have impaired abdominal wall integrity. Also, their use may be difficult in patients with a low pain threshold and psychologically intolerant in terms of the sense of pressure and pain caused by the pressure applied against the abdomen. In our clinic, there was no patient who could not tolerate the compression-induced pressure and pain sensation. However, the AC technique could not be used in one patient with an abdominal colostomy stoma placed in the midline.
The most common side effect of RT applications for the liver is radiation-induced liver disease (RILD). It was first described by Reed et al., and its symptoms include elevated alkaline phosphatase, hepatomegaly, anicteric ascites, increased abdominal girth, and fatigue [26, 27]. It typically occurs within two months of treatment and is closely associated with whole liver radiation at doses of ≥ 30–35 Gy in 2 Gy fractions. In terms of normal tissue tolerances, whole liver radiotherapy up to 30 Gy in 2 Gy fractions was found to be associated with a 5% risk of liver failure within five years, while whole liver radiotherapy up to 40 Gy was associated with a 50% risk of RILD [28]. However, most SBRT studies involve a very low risk of RILD due to appropriate patient selection and strict dose-volume restrictions [29–32]. In our clinic, RILD was not observed in any patient who underwent SBRT with AC.
Limiting the doses to adjacent tissues and organs in SBRT applications for the liver contributes greatly to the management of side effects. Since these structures are non-target and the dose is high, it is necessary to limit the doses to such tissues and organs as ribs, duodenum, small bowel, and great vessels. Previous studies observed grade 1–2 anorexia and nausea when the whole stomach dose was limited to 7–30 Gy and identified more common complaints related to the lesions closer to the stomach. Diarrhea appears to be the most common intestinal toxicity and is usually at an acceptable level. One study reported duodenal ulceration and colonic perforation, but these occurred at intestinal doses above 30 Gy. Skin toxicity was at an acceptable level and limited to erythema and pain, with only one study reporting skin ulceration six months after treatment. General complaints such as fatigue, fever, and chills were common but mild. In all studies, renal, cardiac, esophageal, and spinal cord-related toxicities were low [33–35]. In our clinic, patients who underwent SBRT with AC had very few acute side effects and no chronic side effects.
In our study, the disease control in the intrahepatic area without SBRT (ofLC) was low, and in the intrahepatic area with SBRT (ifLC) it was high. This situation is compatible with the literature and shows that SBRT is an effective treatment. At the same time, it emphasizes that the treatment of liver metastases is multimodal and that the contribution of treatments such as surgery, immunotherapy and chemotherapy is necessary [36–38]. Follow-up of at least six months after SBRT is required to determine ifLC [39]. This is necessary in order to avoid early radiological uncertainties. The patients included in the study had liver metastases of different cancer types. Because of this heterogeneity, the overall survival data were thought to be insignificant and therefore not calculated.
The lack of a control group for comparison is a shortcoming of the study. However, “time to start the treatment” and “number of repositionings” are compatible with the literature. In addition, they do not appear high when compared to patients using other radiotherapy equipment, and especially the “number of repositionings” is significantly lower.
Conclusions
Stereotactic body radiotherapy with AC is a simpler, low-cost, easy-to-apply, and patient-tolerant technique compared to other practices that provide respiratory motion restriction or respiratory compliance. Our study achieved reasonable tumor response rates and a significantly low side-effect profile, suggesting that SBRT with AC is a clinically effective and safe technique. Comparative studies with larger patient series are needed.








