Summary
The current study aimed to determine exercise sensitivity and its associated kinesiophobia and physical activity levels in patients with chronic coronary syndrome (CCS). Forty-three patients and 45 healthy controls were included in this cross-sectional study. Exercise sensitivity, physical activity level, and kinesiophobia were compared. Patients with CCS exhibited fear and sensitivity towards exercise, and these fears contributed to high levels of kinesiophobia and low physical activity levels. Our study fills a critical gap in the literature by directly comparing exercise sensitivity, kinesiophobia, and physical activity levels between CCS patients and healthy individuals. This approach provides valuable insights that can inform future research and clinical practice, keeping the audience informed and engaged.
Introduction
Cardiovascular diseases (CVD) are one of the leading causes of death globally, accounting for 32% of all deaths [1]. The WHO predicts that more than 38% of adults will have one or more forms of CVD [2]. Chronic coronary syndromes, also referred to as stable coronary artery disease (CAD), stable ischaemic heart disease, chronic stable angina, or stable angina pectoris, involve feelings of tightness, heaviness, or pressure in the chest, neck, and jaw [3]. Angina pectoris (AP) is an important symptom of chronic coronary syndromes, and it is seen in 26.8–33% of these patients. AP is sudden, short-lasting, and triggered by diet, emotions, and physical activity. Research shows that AP can occur with stress and psychological distress, can seriously affect the quality of life, and is associated with fear of movement (kinesiophobia) [4, 5]. Additionally, kinesiophobia was detected in different percentages in patients with CAD [6–8]. In the fear-avoidance model, kinesiophobia was initially defined as fear of pain and was changed to “fear of heart attack” in CAD patients. This situation has weakened the effect of “fear of pain” on kinesiophobia in CAD patients to some extent [9]. The expanded version of the fear-avoidance model states that pain resistance should also be taken into account and that this is explained by exercise sensitivity [10, 11].
Exercise is a fundamental component of cardiac rehabilitation (CR) and an essential part of the secondary prevention care process for individuals with CVD. However, low adherence and irregular participation in CR are observed [12]. Evidence shows that fear of physical activity is common in patients with cardiac disease and that this reduces exercise and CR participation [4, 12].
Sensitivity to internal sensations is crucial to avoidant behaviour towards distressing bodily sensations. Cardiac risk factors or diseases can increase anxiety by increasing awareness of physical sensations and making exercise sensitivity dangerous or intolerable [13]. Reducing exercise-related sensory fear is essential for improving exercise participation for CVD prevention and CR [14]. There is a need to determine exercise sensitivity to increase chronic coronary syndrome (CCS) patients’ participation in therapeutic exercise programs. Therefore, the extent and the way in which exercise sensitivity affects physical activity and fear of movement in CVD is unclear. The present study aimed to determine exercise sensitivity and its associated kinesiophobia and physical activity levels in patients with CCS.
Aim
This study sought to fill existing gaps in the literature by demonstrating the status and relationship of exercise sensitivity, physical activity, and kinesiophobia in patients with CCS, compared to healthy controls.
Material and methods
This study was conducted between October 2023 and February 2024 at University’s Department of Cardiology. It received ethical approval from the University’s Non-Interventional Clinical Research Ethics Committee (No: 2023-12). Informed consent was obtained from the CCS patients and healthy controls. The study followed the principles of the Declaration of Helsinki.
This cross-sectional case-control study included 43 patients and 45 age- and gender-matched healthy controls. The inclusion criteria for CCS patients included the following: being 18 years old or above, having a physician-diagnosed CCS with stable angina, having experienced angina pectoris (chest pain or radiating pain) in the past 6 months, and having a stable condition, Participants provided verbal and written informed consent.
The study started by scanning the data files of individuals (both patients and healthy individuals) who had previously applied to the cardiology clinic and participated in scientific studies.
Individuals who met the inclusion criteria and agreed to participate were included. Researchers prepared 10 items to assess exercise sensitivity, and kinesiophobia and physical activity levels were assessed with surveys.
Participants under 18 years old, pregnant, with active infection, known malignancy, lack of consent, acute or chronic renal failure, hypo- or hyperthyroidism, non-atherosclerotic causes of secondary angina, arrhythmia, dilated or hypertrophic cardiomyopathy, heart failure (ejection fraction (EF) < 40%), single functional epicardia vessel, pulmonary hypertension, or acute coronary syndrome were excluded.
For healthy controls, age- and gender-matched individuals with any diagnosed disease, who agreed to participate in the study, were included. Individuals with cardiac, orthopaedic, and neurological diseases were omitted.
Demographic, physical, and physiological characteristics were recorded by asking the participants questions or using data files. The primary outcome measure is exercise sensitivity results. Secondary outcome measures were kinesiophobia and physical activity level results.
The researchers evaluated exercise sensitivity, characterised by cardiopulmonary sensitivity and anxiety about pain/weakness sensations during exercise, using a 10-item questionnaire prepared in a 5-point Likert scale. Items were about fear of cardiopulmonary sensations during training (i.e. blurred vision, angina/tightness, difficulty breathing) and fear of pain/weakness during exercise (Table I). The validity of the questionnaire was assessed by the technique developed by Lawshe [15] and the critical content ratio determined by Ayre and Scally [16]. Five experts participated in the study. An “expert form” was questioned, and the answers were used as a data collection tool. The content validity ratio for the responses of the 5 experts was at least 0.99, with a significance level of p = 0.05. Cronbach’s α, which was used to assess the internal consistency of the validated questionnaire, had a value of 0.874. The total score of the exercise sensitivity questionnaire (ESS) was calculated. The range of the ESS was 10–50.
Table I
Exercise sensitivity items and results between patients and heathy participants
The Tampa Scale for Kinesiophobia for Heart (TSK-H) was used to assess kinesiophobia. The Turkish validity and reliability of the scale in heart patients was performed by Acar et al. [17]. It contains 17 items with a 4-point Likert scoring system (1 = strongly disagree, 2 = agree, 3 = disagree, 4 = completely agree). The total score, ranging from 17 to 68, is calculated after reversing items 4, 8, 12, and 16. A total TKS-H score above 37 indicates that the person has a high level of kinesiophobia [16].
The short-form Physical Activity Evaluation Questionnaire developed by Craig et al. [18] was used to assess the physical activity level. In this survey, physical activities were considered as criteria for at least 10 min at a time. In the last 7 days of the study, duration (min) of vigorous, moderate physical activity, walking, and sitting time (minutes) per day were recorded and calculated [18].
Statistical analysis
Sample analysis: The sample size was determined by the formula ‘’N = 2 × (Zα/2 + Zβ)2 × (p1(1 – p1) + p2(1 – p2))/(p1 – p2)2 [19]. Considering the kinesiophobia results (p2 = 74.5%) of a similar study [8], the ideal sample size was calculated as at least 86 participants (43 participants in each group) with α err prob = 0.05, power (1 – β err prob) = 0.95.
The statistical analyses, conducted using the IBM SPSS Statistics 25.0 package (IBM SPSS Inc., Chicago, USA), revealed significant findings. Normality was checked by using visual (histogram and graphs) and analytical (Kolmogrov-Smirnov) methods. Descriptive statistics included mean values and standard deviations for normally distributed continuous variables, and number (n) and percentage (%) values for categorical variables. Independent sample t-tests (Student’s t-test) were used to compare the normally distributed variables of the CCS patients and healthy controls, and the differences between healthy and CCS patient groups were presented by mean ± standard deviation (x ± SD). The Mann-Whitney U test was performed for the variables that did not comply with normal distribution, and the differences between the groups were shown with median and quartile (IQR, 25–75%) values. Correlations between the ESS and IPAQ, as well as TKS-H, were analysed using Spearman’s correlation analysis because the data met nonparametric assumptions. The strength of the correlations was assessed as follows: 0.00 to 0.25 = negligible or none; 0.25 to 0.50 = fair; 0.50 to 0.75 = moderate to good; and 0.75 to 1.00 = good to excellent [20]. Multivariable linear regression analysis was also used to evaluate the association between exercise sensitivity total score and physical activity total score and Tampa Kinesiophobia Scale total score. The p-values less than 0.05 were considered as significant.
Results
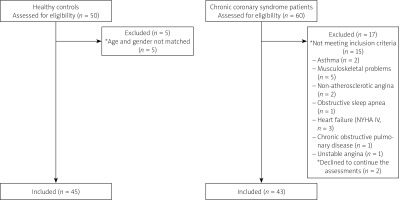
Sixty CCS patients with angina pectoris were enrolled in the study. Of those, 15 did not meet the inclusion criteria: asthma (n = 2), musculoskeletal problems (n = 5), non-atherosclerotic angina (n = 2), obstructive sleep apnoea (n = 1), heart failure (NYHA IV, n = 3), chronic obstructive pulmonary disease (n = 1), and unstable angina (n = 1). During the assessments, 2 CCS patients did not want to continue. For the control group, 50 healthy controls were asked to participate, and 45 were included in the analyses (5 did not match age and gender). Therefore, 43 patients and 45 healthy controls were assessed (Figure 1).
Table II compares the demographic and clinical characteristics of the patients with CCS and healthy controls. Eighty percent of healthy controls and 86% of patients aged 43 to 67 years were male. A total of 51.2% of CCS patients reported experiencing angina during activity, and 3.4% of them suffered angina at rest. The current smoker percentage (p < 0.001), body mass index (BMI) (p < 0.001), primary school education (p = 0.009), and alcohol consumption (p = 0.002) results were higher in patients compared to healthy controls. 62.8% of the CCS patients had a history of hypertension, 48.8% had a history of diabetes, and 9.3% had dyslipidaemia. Although patients had a higher percentage of physical activity (PA) history, statistically similar PA histories were found between patients and healthy controls (p = 0.51).
Table II
Demographic and clinical characteristic of the CCS patients and healthy participants
Exercise Sensitivity Questionnaire items and results between CCS patients and healthy controls are shown in Table I. More than half of the patients disagreed with the statement that ‘feeling pain (p = 0.001), depletion of energy (p = 0.033), and fatigue (p = 0.007)’ during exercise scared them. Although 65.1% of CCS patients accepted that the blurred vision that may occur during exercise scares them, no statistically significant differences in this item were found between the groups (p = 0.08).
Patients mostly accepted that during exercise ‘feeling chest pain (p = 0.021), chest tightness (p = 0.037), dizziness (p = 0.046), palpitations (p = 0.001), dyspnoea (p = 0.008), and fainting (p = 0.001)’ would frighten them, and the results were statistically different between healthy controls and CCS patients. 68.9% of healthy controls strongly agreed that feeling faint during exercise would be frightening, and 65.1% of patients with CCS agreed. A significant difference was found between the 2 groups in terms of this item (p = 0.001).
Exercise sensitivity, kinesiophobia, and Physical Activity Scores between patients with CCS and healthy controls are shown in Table III. Exercise Sensitivity Score questionnaire (ESS) results were statistically lower in patients compared to healthy controls (33.65 ±5.83 versus 37.40 ±7.38, p = 0.010). TSK-H was higher in patients compared to healthy controls, and the difference was statistically significant between the groups (p = 0.007). Although the percentage of individuals with high kinesiophobia was found to be statistically similar in both groups (p = 0.06), 58.1% of patients had a high level of kinesiophobia. Physical activity levels were lower in CCS patients than in healthy controls (p < 0.001), and 58.1% of patients were minimally active (p = 0.010).
Table III
Exercise sensitivity, kinesiophobia and physical activity scores between CCS patients and healthy participants
The ESS was significantly associated with the IPAQ score, showing a fairly negative correlation (r = –0.360; p = 0.018). It was also moderately positively correlated with the TKS-H score (r = 0.529; p < 0.001) in patients with CCS.
Multivariate linear regression analysis was performed to predict the exercise sensitivity variable using physical activity and kinesiophobia variables (Table IV). Exercise sensitivity explained 25% of kinesiophobia and physical activity according to linear regression analyses. TKS-H = β0 + β1 × (exercise sensitivity), a one-point increase in exercise sensitivity (β1 = 0.33) is associated with a 0.33-point increase in TSK-H, with a 95% confidence interval of 0.206 to 0.457.
Discussion
To the best our knowledge, this is the first study to compared exercise sensitivity, kinesiophobia, and physical activity in CCS patients and healthy controls. The important findings of the present study are as follows: 1) The Exercise Sensitivity Score was higher in the CSS group. Patients disagreed that during exercise ‘feeling pain, depletion of energy, and fatigue’ would scare them. However, CCS patients mostly accepted that during exercise ‘feeling chest pain, chest tightness, dizziness, palpitations, dyspnoea, and fainting’ would frighten them. All the participants stated that the blurred vision that may occur during exercise scares them. 2) The Tampa score was higher in CCS patients compared to the healthy controls. The high kinesiophobia level was similar between the patients and healthy controls. More than half of the patients had high-level kinesiophobia. 3) The physical activity level was lower in CCS patients than in healthy controls, and more than half of the patients were minimally active. 4) The exercise sensitivity score showed a somewhat negative correlation with the IPAQ score and a moderate positive correlation with the TSK-H score in CCS patients. Furthermore, regarding these findings, it was determined that the kinesiophobia independent variable predicted the exercise sensitivity variable positively in patients with CCS.
Emotions regulate the mind and body, aiding survival and well-being, and they are vital in sustaining long-term exercise habits. Exercise can evoke a wide range of emotions, from anger to euphoria. Positive emotions like joy reinforce exercise motivation, while negative emotions such as pain can deter it, especially when starting a new routine. However, with consistent exercise, negative emotions can turn positive, boosting motivation. A positive mood encourages more exercise, further enhancing mood and creating a beneficial cycle [21]. Most of the CCS patients declared that during the exercise, ‘having chest pain, feeling tingling and dizziness, having increasing heart rate and palpitations’ would scare them, according to the current study. However, they disagreed that during the exercise, ‘feeling pain, depletion of energy, and fatigue’ would scare them. Healthy controls declared similarly about exercise sensitivity but with different degrees, in the present study. Determining exercise sensitivity in transitioning from a passive to an active lifestyle may be necessary, especially when starting routine exercise [22]. However, strict adherence to the exercise program could quickly turn even the negative emotions initially caused by exercise into positive ones, thereby increasing motivation to exercise. Moreover, a positive mood is associated with the likelihood of exercising more, which yields better results [6, 21–23]. In this respect, we believe that the results of the present study may be useful for CCS patients with low exercise participation rates.
Significant unpleasant sensations, such as angina pectoris during physical activity, are linked to high levels of kinesiophobia (fear of movement) [5]. Over 76% of patients with coronary artery disease (CAD) exhibited high levels of kinesiophobia [6]. These patients tended to be less physically active [7]. Another study concluded that more than 70% of CAD patients revealed high levels of kinesiophobia (TSK > 37 points) [7]. Baykal Şahin et al. found that a high level of kinesiophobia was present in 74.5% of the CAD patients, and the mean TSK-heart score was 41.4 ±6.2 [8]. A recent study’s results showed that the total score of kinesiophobia in CAD patients with AP was 40.80 ±6.65, and the vast majority of patients had moderate to high levels of kinesiophobia (75.7%) [4]. A high level of kinesiophobia was present in 87.2% of coronary artery disease patients, with those having a history of myocardial infarction (MI) showing higher levels compared to those without such a history [24]. Kinesiophobia was initially defined in the fear-avoidance model by Lethem et al., which centres on the fear of pain [25]. Bäck et al. suggested that for CAD patients, the term ‘fear of pain’ should be changed to ‘fear of heart attack’. This change somewhat lessened the impact of ‘fear of pain’ on kinesiophobia in these patients [9]. In the current study, the Tampa score was higher in CCS patients compared to the healthy controls. Although the percentage of high kinesiophobia levels was found to be statistically similar in both groups, contrary to the previous study [6], 58.1% of patients had a high level of kinesiophobia, but similarly, the physical activity level was lower in CCS patients than in healthy controls in our study. According to a previous study evaluating the kinesiophobia and benefits/barriers of exercise in cardiovascular diseases, kinesiophobia was correlated with factors such as perceiving the exercise barriers of physical effort [23]. Also, perceiving the exercise benefits from the psychological perspective may provide new perspectives for prevention and interventions related to kinesiophobia in patients with CVD [23]; in this study, the perception of exercise benefits/barriers and kinesiophobia among CVD patients and their interactions with kinesiophobia were examined. In our study, the sensitivity of exercise, kinesiophobia, and physical activity interactions in CCS patients and healthy controls were compared comprehensively, and we found that feeling dizzy during exercise (67.4%) would mostly scare the CCS patients. In addition, exercise sensitivity explained 25% of kinesiophobia and physical activity according to linear regression analyses in the present study. Exercise sensitivity and barriers to exercise need to be determined in advance to increase participation in CR programs. It is recommended that future studies evaluate these parameters and determine their cutoff value in CVD diseases.
Low-level physical activity in patients with CSS is explained by several factors: fear of exacerbating symptoms such as a heart attack [26], fear of movement (kinesiophobia) [6], physical limitations which can cause fatigue, shortness of breath, and chest pain [27], lack of confidence [28], interceptive sensitivity and insufficient guidance emotional factors such as depression, anxiety, stress; and social support that lead to lack of support from family, friends, or healthcare providers [29].
39.5% of CCS patients with stable angina were inactive, and 58.1% were minimally active in the present study. Although the patients were minimally active, the patients’ physical activity levels were lower than those of healthy controls. Previous studies have shown that physical activity sensitivity has been identified as a significant risk factor for physical inactivity among individuals experiencing pain [30, 31]. Poor physical performance could be attributed to high levels of activity-related pain [30]. Furthermore, pain catastrophising has been identified as a significant predictor of physical activity sensitivity [31]. These findings suggest a strong relationship between exercise sensitivity and pain-related factors. However, Reid stated that sensitivity to physical activity was common and linked to negative outcomes, such as decreased physical activity, even in older adults without chronic pain [32]. Therefore, low physical activity levels may be associated with exercise sensitivity and fear of movement. This suggests that physical activity sensitivity may be a significant issue for public health. Notably, a one-point increase in exercise sensitivity was associated with a 0.33-point increase in TKS-H in the current study. These findings suggest a meaningful relationship between exercise sensitivity and both kinesiophobia and physical activity levels, emphasising the importance of addressing exercise sensitivity in interventions aimed at improving physical activity and reducing kinesiophobia in CCS patients. Increased awareness of bodily sensations due to cardiac conditions can increase anxiety, leading to catastrophising angina pain, and making patients more likely to misinterpret normal exercise sensations as dangerous. This can be avoided by assessing the exercise sensitivity beforehand, as in our study. Thus, the design of targeted interventions to improve overall health may be more effective by measuring exercise sensitivity in stable angina patients.
While our study sheds light on exercise sensitivity, kinesiophobia, and physical activity levels in patients with CCS, certain limitations must be acknowledged. Objective physical activity assessments such as accelerometers could have been used to clarify the possible relationship between exercise sensitivity, physical activity level, and kinesiophobia. The study’s limited sample size, single district, and hospital focus, along with the use of convenience sampling, may result in bias and lack of representativeness. The duration of coronary artery disease and the year of onset of angina pectoris could be obtained. Because CCS increases awareness of bodily sensations, anxiety status in individuals with CCS should also be evaluated in future studies. The current study is the first to show the change in exercise sensitivity between CCS patients and healthy controls. Another strength of our study is its inclusion of a homogeneous patient group. Strictly following an exercise program when transitioning from a sedentary to an active lifestyle can transform initial negative feelings about exercise into positive ones, boosting motivation. Therefore, we believe the results of this study may benefit CCS patients with low exercise participation rates.
Conclusions
This study focused on comparing the exercise sensitivity and its relationship with kinesiophobia and physical activity levels between chronic coronary syndrome patients with angina pectoris and healthy controls. Patients with CCS were afraid of situations such as blurred vision, chest pain, chest tightness, dizziness, palpitations, dyspnoea, and fainting that might occur during exercise. The patients had high levels of kinesiophobia, and physical activity levels were minimal. Exercise sensitivity was positively correlated with kinesiophobia and negatively with physical activity level. According to linear regression analyses, exercise sensitivity explained 25% of kinesiophobia and physical activity. Taking into consideration exercise sensitivity, kinesiophobia, and physical activity level may be effective in organising successful therapeutic programs and increasing participation in exercises in patients with CCS. This approach can provide valuable insights that can guide clinical practice and keep the audience informed and engaged. It is recommended that future studies evaluate exercise sensitivity and barriers and determine the cutoff value of these parameters in CVD diseases.