Introduction
Cirrhosis, which results from chronic liver inflammation and subsequent fibrosis, and affects the liver architecture, is a global problem that promotes the formation of regenerative nodules; this can ultimately lead to portal hypertension and organ failure [1]. Nonalcoholic fatty liver disease (NAFLD) is probably the most common cause of chronic liver disease worldwide, with a prevalence of up to 30% in the general population. This condition is defined based on fat infiltration into the liver in the absence of known causes of hepatic steatosis, such as excess alcohol consumption, chronic viral hepatitis, autoimmune hepatitis, or medications [2]. NAFLD-related cirrhosis has recently become the leading indication for liver transplantation in Europe, surpassing chronic viral hepatitis [3].
In patients with cirrhosis, oesophageal varices (OV) are one of the main complications associated with portal hypertension, whose development marks an important transition stage and identifies a subset of patients at risk of decompensation due to variceal bleeding [4, 5]. Approximately 90% of patients with portal hypertension may develop OV at any time during their lifetime, and approximately 33% of these OV may bleed [6]. Despite advancements in the medical and endoscopic treatment of variceal haemorrhage over the last 2 decades, the mortality of patients with variceal bleeding remains approximately 25% after a bleeding episode [7]. Thus, early identification of OV, which allows physicians to establish timely prophylactic measures for preventing first bleeding, represents an essential part of the diagnostic workup of patients with cirrhosis [8].
Upper gastrointestinal (GI) endoscopy is the gold standard for both identifying and classifying the grade of OV while serving as a therapeutic intervention. However, this procedure is invasive and unpleasant, with questionable cost effectiveness and practicality for universal OV screening [9]. These health care resource-related limitations and the progressively increasing workload on endoscopy units the world over has prompted many researchers to seek parameters that can predict OV noninvasively [9–12]. Recent criteria proposed in Baveno VI suggest that screening endoscopy can be successfully avoided in patients with compensated advanced chronic liver disease (cACLD) who have a liver stiffness (LS) measurement of < 20 kPa on transient elastography and a platelet (PLT) count of > 150 × 109/l [11]. The application of an expanded Baveno VI criteria with different PLT count (> 110 × 109/l) and LS measurement (< 25 kPa) thresholds can spare even more endoscopies while carrying minimal risk for missing OV that need treatment in only 1.6% of patients fulfilling the criteria and 0.6% of the general population of patients with cACLD [12].
Aim
The current study aimed to compare the efficacy of various noninvasive methods published in the literature, including Baveno VI, expanded Baveno VI, and PLT count/spleen diameter ratio, for the noninvasive prediction of OV requiring treatment in patients with NAFLD-associated cACLD. We also aimed to determine whether a superior prediction tool can be developed using the combination of the abovementioned factors, such as PLT count/(spleen diameter + LS), with and without adjusting for albumin level.
Material and methods
Study design and patients
This retrospective observational analysis evaluated the electronic medical records of all patients diagnosed with NAFLD-related liver fibrosis who visited the Gastroenterology Clinic of Tawam Hospital, United Arab Emirates, and underwent transient elastometry (Fibroscan, Echosense, Paris) between June 2017 and May 2022 (past 5 years). This study was registered with the clinical trial database ClinicalTrials.gov (identifier no. NCT05485714), and ethical approval was obtained from Tawam Human Research Ethics Committee (approval number: MF2058-2022-856).
Patients who fulfilled the following inclusion criteria were included: age ≥ 18 years; either male or female patients without any clinical signs of severe decompensation; Fibroscan-reported LS measurement of ≥ 7 kPa (indicative of fibrosis stage ≥ F2); ultrasound findings showing fatty liver changes confirmed by an experienced ultrasonologist; and stable vital signs (without severe encephalopathy or evidence of hematemesis or melena). Additionally, we only selected patients who underwent upper GI endoscopy and relevant blood tests within 12 months from the Fibroscan LS measurement to avoid interpretation bias due to disease progression. Any patient with active alcohol consumption within the past 6 months; history of excess alcohol consumption within the past 5 years; evidence of other aetiologies for liver cirrhosis, such as infection with hepatitis viruses or other causes (e.g. primary biliary cirrhosis or autoimmune hepatitis); and HIV infection or comorbid liver or biliary diseases were excluded. Patients who had undergone prior endoscopy with evidence of intervention, such as band ligation or sclerotherapy, or any surgery/procedure for portal hypertension were also excluded from this study.
After evaluating the records of 1258 patients, we found that only 986 had completed clinic follow-up and blood tests. From these 986, only the patients who underwent screening endoscopy for OV were identified (n = 73). All data pertaining to presenting illness, documented physical signs, and biochemical workup, including PLT count, albumin, and examinations such as abdominal ultrasound for the presence of ascites and bipolar splenic diameter measurement, as well as data for LS measurement and upper GI endoscopy results, were obtained. OV were categorised into small and large, according to the Baveno recommendations [11]. The expanded Baveno VI criteria, Baveno VI criteria, and PLT count (mm–3) to bipolar spleen diameter (mm) ratio was calculated in all selected patients and compared according to endoscopy results.
Criteria and formulas
OV prediction in patients with cACLD was determined using the Baveno VI criteria, the expanded Baveno VI criteria, and the PLT count (mm–3) to spleen diameter (mm) ratio.
We developed and proposed a new formula that included a combination of previously published predictors like PLT count to spleen diameter ratio and LS: (PLT count/Spleen diameter) + LS. Subsequently, liver function was considered using albumin levels, which were added to this formula: (PLT count/Spleen diameter) + LS × (40 – Albumin) to see if it gave any further predictive value.
Statistical analysis
Continuous data were presented as median and interquartile range (IQR), whereas categorical data were presented as absolute values and percentages. Fisher’s exact test or Pearson χ2 test was used to compare the categorical variables according to the sample size. To assess the clinical and biochemical disease activity index, Wilcoxon’s test for paired data was applied. We used the Mann-Whitney U test to compare between the groups. A p-value of < 0.05 in a two-tailed test was considered to indicate statistical significance. Receiver operating characteristic (ROC) curves were constructed to identify optimal cut-off values for predicting the presence of OV and large OV [13]. The Youden index was used to identify the optimal cut-off point for each score. Comparisons of AUCs were carried out using the method proposed by DeLong et al. [14].
Results
A total of 73 patients satisfied the inclusion criteria and were included in this study. Their median age was 60 (IQR: 42–67; range: 19–88) years. Among these 73 patients, 41 (56.2%) were male and 48 (65.8%) were born in the Emirates.
Overall, 67 (91.8%) patients had preserved liver function (55 (75.3%) and 12 (16.4%) patients had Child-Pugh classes A5 and A6, respectively), whereas only 5 (6.8%) and 1 (1.4%) patient/s had Child-Pugh classes B and C, respectively. Table I summarises the general characteristics and liver function parameters of patients.
Table I
General characteristics of the study population
The median spleen diameter was 107 (IQR: 94.2–133) mm, and transient elastometry showed a median LS of 10.2 (IQR: 8.2–16.6) kPa.
Table II details the presence of both OV and large OV in patients stratified according to different criteria based on LS, PLT count, spleen diameter, and liver function. Overall, 18 of 73 (24.7%) patients had OV, among whom 8 had large OV (11.0%).
Table II
The presence of oesophageal varices and large varices stratified according to different noninvasive diagnostic criteria
Area under the receiver operating characteristic curve analysis of Baveno VI for predicting the presence of OV showed an area under the curve (AUC) of 0.83 with 95%CI (95% confidence interval) of 0.71–0.94; sensitivity (Se)/specificity (Sp) of 83.3/81.8; positive predictive value (PPV)/negative predictive value (NPV) of 60.0/93.7; and likelihood ratio of a positive test (LR+)/likelihood ratio of a positive test (LR−) of 4.58/0.2), while the AUC for Baveno VI expanded criteria for predicting the presence of OV was 0.79 (95% CI: 0.65–0.93; Se/Sp, 66.7/90.9; PPV/NPV, 70.6/89.3; LR+/LR−, 7.33/0.37). Moreover, the same criteria for predicting the presence of large OV showed an AUC of 0.80 (95%CI 0.65–0.95; Se/Sp 87.5/72.3; PPV/NPV 28.1/97.9; LR+/− 3.16/0.17) and 0.79 (95%CI 0.61–0.98; Se/Sp 75/83.1; PPV/NPV 35.4/96.4l; LR+/− 4.44/0.3) for Baveno VI and Baveno VI Expanded, respectively.
The (PLT count/Spleen diameter) formula had an AUC of 0.84 (0.73–0.96) with a cut-off of ≤ 1472 (Se/Sp 83.3/78.2; PPV/NPV 56/93.5; LR+/− 3.82/0.21) for predicting the presence of all OV and an AUC of 0.94 (0.87–1) with a cut-off of ≤ 933 (Se/Sp 87.5/90.8; PPV/NPV 54/98.3; LR+/− 9.51/0.14) for predicting the presence of large OV (Figures 1 and 2).
Figure 1
Area under the receiver operating curve analysis of different criteria for predicting the presence of oesophageal varices of any grade in patients with compensated advanced chronic liver disease
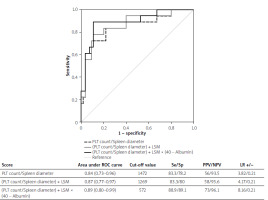
Figure 2
Area under the receiver operating curve analysis of different criteria for predicting the presence of large oesophageal varices in patients with compensated advanced chronic liver disease
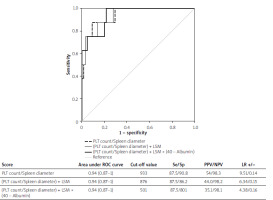
The (PLT count/Spleen diameter) + LS formula had an AUC of 0.87 (0.77–0.97) with a cut-off of ≤ 1269 (Se/Sp 83.3/80.0; PPV/NPV 58/93.6; LR+/− 4.17/0.21) for predicting the presence of all OV and an AUC of 0.94 (0.87–1) with a cut-off of ≤ 876 (Se/Sp 87.5/86.2; PPV/NPV 44.0/98.2; LR+/− 6.34/0.15) for predicting the presence of large OV (Figures 1 and 2).
The (PLT count/Spleen diameter) + LS × (40 – Albumin) formula had an AUC of 0.89 (0.80–0.99) with a cut-off of ≤ 572 (Se/Sp 88.9/89.1; PPV/NPV 73/96.1; LR+/− 8.16/0.12) for predicting the presence of all OV and an AUC of 0.94 (0.87–1) with a cut-off of ≤ 501 (Se/Sp 87.5/80.0; PPV/NPV 35.1/98.1; LR+/− 4.38/0.16) for predicting the presence of large OV (Figures 1 and 2).
The pairwise comparison among AUCs showed that the (PLT count/Spleen diameter) + LS × (40 – Albumin) formula showed a significantly higher accuracy for predicting the presence of any grade OV other than both Baveno VI and expanded Baveno VI criteria (differences in AUC, 0.07 and 0.11; p = 0.030 and p = 0.050, respectively). Moreover, Baveno VI and expanded Baveno VI criteria were inferior to the (PLT count/Spleen diameter) (differences in AUC, 0.14 and 0.15; p = 0.0001 and p = 0.010, respectively), (PLT count/Spleen diameter) + LS (differences in AUC, 0.14 and 0.15; p = 0.0005 and p = 0.006, respectively), and (PLT count/Spleen diameter) + LS × (40 – Albumin) (differences in AUC, 0.15 and 0.16; p = 0.0002 and p = 0.006, respectively) formulas in predicting the presence of large OV.
Overall, the numbers of spared endoscopies were 47 (64.4%) and 54 (74.0%) using the Baveno VI and expanded Baveno VI criteria, respectively, and 59 (80.8%), 60 (82.2%), and 63 (86.3%) using (PLT count/Spleen diameter), (PLT count/Spleen diameter) + LS, and (PLT count/Spleen diameter) + LS × (40 – Albumin) formulas, respectively.
Discussion
To date, hepatic venous pressure gradient has been considered the gold standard for the diagnosis of clinically significant portal hypertension (CSPH); however, it is an invasive test that requires an equipped centre and expert staff [5, 15, 16]. Several noninvasive methods have been used to stratify and predict the presence of CSPH and OV development in patients with CLD and safely decrease the need for endoscopy [17]. Moreover, the new Baveno consensus research agenda focuses on the need for validating and refining these noninvasive tools for detecting CSPH in patients with NAFLD-associated cACLD, because the current evidence has no standard recommendations other than well-established aetiologies.
The present study weighed the different combined biochemical and radiological parameters in predicting the presence and severity of OV in patients suffering from NAFLD-associated cACLD. Overall, both the Baveno criteria (i.e. Baveno VI and expanded Baveno VI) and PLT-to-spleen diameter ratio, which also incorporates LS and albumin measurements, were effective in predicting the presence of varices of any size, with an AUC of up to 0.8 on AUROC analysis. Although the PLT count/spleen diameter ratio has been used to predict OV in patients with CLD secondary to infectious aetiologies like chronic hepatitis C and schistosomiasis, it has not been specifically used in patients with NAFLD [16, 17]. In fact, all Baveno criteria and PLT count/spleen diameter ratios, albeit consolidated in CLD, have not been validated in the NAFLD population [11, 12, 18]. Interestingly, our proposed formula appears to be effective in this population of patients, with the addition of LS and albumin measurements seemingly having better accuracy compared to PLT count/spleen diameter ratio alone.
Indeed, when comparing the Baveno VI and expanded Baveno VI criteria with (PLT count/Spleen diameter) + LS × (40 – Albumin), the latter appears to better predict the presence of any size and large OV. We believe that all parameters included in the proposed formula are commonly available during clinical evaluation and could represent a simple and uncostly tool for stratifying CLD patients in actual clinical practice with a higher accuracy than previously known criteria [18, 19].
Moreover, these formulas could decrease the financial burden of rural care centres with limited financial and logistic resources by reducing the endoscopy costs. In fact, the present findings highlighted that a significant number of endoscopies could be avoided by using all scores proposed. Moreover, several studies have already demonstrated that the application of noninvasive predictive tools, such as the PLT count/spleen diameter ratio, for the detection of OV would offer more cost-effective approach than would the “scope all strategy” [18, 20].
We believe that the incorporation of LS and serum albumin measurements into the previously validated PLT/spleen diameter ratio is of interest and is supported by both clinical and statistical evidence. From a clinical perspective, LS measurements could serve as an additional parameter reflecting liver fibrosis and inflammatory involvement typical of nonalcoholic steatohepatitis pathogenesis and should not be influenced by other causes of thrombocytopaenia or splenomegaly, such as haematological disorders [21]. Moreover, serum albumin levels could provide objective and reliable information on liver function in patients with overall preserved hepatic compensation [22]. From a statistical viewpoint, the (PLT count/Spleen diameter) + LS × (40 – Albumin) formula seems to be the most effective and accurate tool for predicting the presence of varices and large varices based on AUROC analysis.
This study has certain limitations. First, it is a retrospective study with a limited sample size. Moreover, although transient elastography has become a useful technique in actual clinical practice, its reliability in the NAFLD population has not yet been confirmed owing to the variable results caused by several factors, such as liver steatosis, inflammation, obesity, food intake, and cholestasis [23, 24]. However, we could establish a simple formula based on noninvasive tests to increase the negative predictive value of OV presence in patients with NAFLD-associated cACLD. Further larger-scale studies are warranted to confirm the wider applicability of this newly proposed formula.
The presence of CSPH and OV in patients with NAFLD and CLD can be safely assessed using noninvasive tools such as Baveno VI, expanded Baveno VI, and PLT count/spleen diameter ratio. A superior predictive value can be easily achieved by considering LS and serum albumin measurements along with the PLT count/spleen diameter ratio.










