Introduction
Allergic asthma (AA) is a relatively common disease in clinical practice, which poses a serious threat to health of children [1]. Relevant epidemiological surveys suggest that 300 million people suffer from AA worldwide, and relevant domestic studies show that about 7.57–48% of children have suffered from AA once in China. With rapid development of the modern society and economy, the prevalence of AA increases year by year due to environmental destruction, especially in children [2]. The causes of such a phenomenon are complex and varied, among which allergic reaction is the major factor leading to asthma. Previous studies have illustrated that about 30–90% of children with AA will develop allergic rhinitis (AR), and most AA cases are caused by AR. This increases the frequency and possibility of paediatric asthma attacks, and thus brings a huge economic pressure to patients and their families [3]. Because AR and AA are similar in pathogenic factors, pathological mechanisms, influencing factors, and more aspects, AR should be treated clinically together with the treatment of AA, which is called “one airway, one disease” theory [4]. There is a close relation between AR and AA. Compared to normal healthy children, children with AA are more likely to suffer from AR.
The clinically known combined allergic rhinitis and asthma syndrome is a clinical manifestation of inflammation caused by respiratory allergies. The severity of symptoms is also related to the type and number of allergens in patients [5]. When allergic inflammation occurs in the nasal cavity, a small number of helper T cells will transfer into bone marrow and stimulate the production of various cytokines, such as mast cells and inflammatory factors. Then these cytokines enter the human body and selectively infiltrate nasal mucosa and lungs, further aggravating respiratory inflammation, thus inducing an allergic reaction and causing related clinical manifestations in patients. Simultaneously, it also increases the content of adhesive molecules in nasal mucosa and bronchial mucosa, thus aggravating patients’ symptoms [6]. AA accompanied with AR is a respiratory disease with high morbidity, which is difficult to treat among paediatric diseases, having a poor prognosis and easy recurrence. It usually results from patients’ autoimmune dysfunction, generally manifested as fibre airway hyperresponsiveness and reversible airway obstruction. Among various factors inducing asthma, allergy is the most vital one [7]. In recent years, the understanding of the immune mechanism of asthma has been gradually improved. It has been discovered that interleukin (IL) and T cells play important roles [8]. For AA, an immune response is mainly produced by the body’s immune system to the surrounding environment. Some studies believe that an abnormal increase in the regulatory T cells (Tregs) level is the main cause of asthma. Under a stimulation, CD4+T cells generate and increase Th2 cells, which stimulates an increase in IL secretion and stimulates the secretion of immunoglobulin E (IgE) by B lymphocytes, leading to airway inflammation [9].
However, as scientists continue to explore, some scholars think that Th1/Th2 imbalance cannot fully explain the pathological mechanism of the asthma attack. Previous studies have also proved that CD4+CD25 Tregs are an important part of human peripheral blood CD4 cells. As early as in the 1990s, some scholars discovered CD4+CD25 Tregs in CD4 cells, accounting for about 5–10% of CD4+T cells; and this could regulate the activity of CD25 cells [10]. The body’s immune system is stabilized through central tolerance and peripheral tolerance, and the cell subsets of T lymphocytes have regulatory ability to suppress immune responses of autoantigens. CD4+CD25high subset has regulatory activity, and CD4+CD25high Tregs can regulate secretion of Foxp3, CTLA-4, and other factors. However, CD25 is not only contained in Tregs, but also partly appears in memory-activated T cells, which lacks specificity. Fox transcription factors are important in cellular development, and Foxp3 is one of Fox transcription factors. Currently, the literature has shown that Foxp3 is a marker of CD4+CD25 Tregs, which is closely correlated with development and function of regulatory Tregs. Foxp3 can regulate immune responses, and the specific expression was not affected by the activation state; it was expressed in Tregs, but not in T cells, B cells, and other cells [11]. During an asthma attack, Tregs secrete a variety of cytokines, such as IL-10 and transforming growth factor β1 (TGF-β1), which play an immune role through the transmission of inflammatory cells. It can also inhibit T cell production, reduce IgE secretion, and induce apoptosis of eosinophils. In different stages of AA, it is always important to analyse the differences among effector cytokines, chemokines, and more for clinical prevention and treatment of asthma [12].
Aim
This study investigates the clinical significance of IL-10, TGF-β1, and CD4+CD25 Tregs in children with AR accompanied by AA, who have received indications for immunotherapy. Focusing on paediatric patients with this specific combination of respiratory conditions, the research aims to analyse changes in these cytokines over the course of treatment. Notably, the study challenges conventional theories by emphasizing the importance of CD4+CD25 Tregs in understanding the immune mechanisms underlying asthma attacks, going beyond the traditional Th1/Th2 imbalance explanation. The longitudinal approach involves comparing indicators between patients and healthy children, providing data references for future clinical treatment or evaluation of AA accompanied by AR. The research novelty lies in its specific clinical focus, inclusion of immunotherapy indications, and its potential to contribute valuable insights to the management of these respiratory conditions in children.
Material and methods
Research subjects
Eighty children suffering from AA accompanied with AR, who received immunotherapy indications in the Department of Paediatrics of the hospital from May 2020 to May 2023, were selected into the experimental group (EG). Inclusion criteria were made up of the following: (1) children were diagnosed with AR with AA through clinical symptoms; (2) children aged 3–14 years old; (3) children with a medical history of AA accompanied with AR of more than 1 year, and the number of acute exacerbations in the previous year ≥ 1; (4) the included children did not participate in other experiments. Exclusion criteria consisted of: (1) children with other respiratory diseases; (2) children with pulmonary diseases, such as active tuberculosis and pneumothorax; (3) children who received other related treatments for acute asthma in the month before this experiment; (4) children who suffer from heart diseases, cancers, or other serious diseases; (5) children with a history of an anaphylactic shock; (6) children who suffer from mental disorders; (7) children who had received immunotherapy in the past.
Another 40 normal children who underwent physical examination in the hospital during the same period were included in the control group (CG). All the subjects included in the study and their families agreed to participate in the study voluntarily and signed the informed consent forms.
Diagnostic criteria
The clinical diagnostic criteria for AA were as follows.
(1) Frequent wheezing, chest distress, shortness of breath, and coughing occurring after exposure to allergens, strenuous exercise, or other stimuli. (2) When the symptoms were mild, patients could be relieved by themselves; when the symptoms became severe, they must be relieved by treatment. (3) Coughing, cough asthma, and chest distress and shortness of breath caused by other respiratory diseases were excluded. (4) Auscultation during the attack showed a scattered or diffuse wheezing sound in both lungs during breathing, with a relative increase in expiratory time. (5) If a child did not present with obvious symptoms and signs, but had one or more of the following, it could also be diagnosed: a) Positive bronchial dilation test; b) Positive bronchial provocation test or exercise test; c) The intraday variation rate or diurnal fluctuation rate of maximum expiratory flow increased.
The clinical diagnostic criteria for AR were referred to in the latest edition of the Chinese Guidelines for Diagnosis and Treatment of Allergic Rhinitis [13].
Therapeutic methods
In this work, standardized immunotherapy methods were adopted. Alutard, mite allergen product, was selected to treat the children. For the therapeutic method, the distal 1/3 of the upper arm of these children was disinfected and subcutaneous injection was given once a week. The dose was gradually increased according to children’s condition until the maximum dose was reached at the 15th week. After that, the dosage remained unchanged, and the medication interval was gradually prolonged until every 8 weeks.
Serological tests
(1) Specimen collection: Peripheral venous blood (about 5 ml of venous blood from the upper elbow) was collected before and after treatment. Serum: venous blood samples were collected in standard test tubes, naturally coagulated at room temperature for at least 30 min and then centrifuged at 1,000 g for 10 min. Next, the separated serum was taken for testing, or stored in separate packages at –20°C. Freezing and thawing for more than two times should be avoided. Frozen samples were thoroughly melted and mixed before use, and particles removed by centrifugation. Plasma: venous blood samples were collected with an Ethylene Diamine Tetraacetic Acid (EDTA) anticoagulant tube and centrifuged at 1,000 g for 10 min. The separated plasma was taken for testing or stored separately at –20°C. Freezing and thawing for more than two times should be avoided. Frozen samples were thoroughly melted and mixed before use, and particles removed by centrifugation.
(2) Precautions: As the child’s family was asked, the child’s general situation, including allergic history, disease severity, and surgical contraindications, were understood. The surgical steps were expounded to the child and his/her family, to get their cooperation. It was checked that reagents and first aid facilities were available. Iodine was used to disinfect the volar side of the child’s forearm. When dry, a mark was made. A drop of positive and negative reagents was applied to sterilized sterile skin, and an allergen solution was added. Each reagent was added with spacing 2 cm apart to avoid swelling, deformation, and skin changes. Then, the allergen was punctured and the standard needle was inserted into dermis so as to avoid mutual contamination. An electromagnetic needle was applied at a time, and the results were observed for 15 min. The child’s family was informed to be quiet while waiting, random movement and scratching were also avoided. The results of the skin spot test were based on the size of the wheal. To avoid allergic reactions in children during the test, the treating doctor should be present for observation during the experiment. In case of allergic reactions, immediate treatment should be provided; in case of severe allergic reactions, intramuscular injection of epinephrine hydrochloride should be administered, meanwhile oxygen or glucocorticoid should also be given. In the study here, no allergic reaction was found in these children.
(3) Evaluation indicators:
Flow cytometry assay: ① 100 µl of anticoagulant whole blood was taken, and 20 ml of anti-CD4-FITc and anti-CD25-Pe monoclonal antibodies were added, respectively. Isotype pairs were set in parallel, and the reaction was performed for 30 min at room temperature, away from light. ② After addition of 1 ml of red blood cell lysate, it was placed at room temperature and away from light for 10 min, and then shaken and mixed well. ③ After centrifugation at 1,500 rpm/g for 5 min, the supernatant was discarded, and the residual was washed once with 2 ml of phosphate buffer saline (PBS). Next, the cells were re-suspended with 400 µl of PBS. ④ CD4+T cells were obtained by staining the CD4 molecule and setting the SSC parameter. The fluorescence threshold was defined by the fluorescence intensity of homologous pairs, and the CD4+CD25 Treg cell population in the experimental tube was determined. The average fluorescence intensity of CD25 was greater than 70 as the high expression standard, and CD4+CD25highT cell subsets were determined. The results were expressed as the percentage of CD4+CD25+T cells and CD4+CD25highT cells in CD4+T cells.
In addition, enzyme-linked immunosorbent assay (ELISA) was used to detect the levels of TNF-β and IL-10 in serum of all subjects.
Before use, all reagents were naturally restored to room temperature for use. 12 cytokine calibrators produced by Qingdao Riskell Biotechnology Co., LTD were selected as calibration products (Registration Certificate No. Lu Wu Admission 20202400205). ① Before the microsphere was prepared for the experiment, the microsphere was scrolled for 30 s, gently blew with a pipette gun for about 30 times, and then scrolled again for 30 s when in use. ② Preparation of the washing buffer: after 10× washing buffer was restored to room temperature and all salts were dissolved, 2 ml 10× washing buffer was added to 18 l of deionized water. The diluted washing buffer can be stored at 2–8°C for 1 month. Â Preparation of matrix B: the lyophilized powder glass bottle of matrix B was slowly rotated to half open; 5 ml of the experimental buffer was added into the glass bottle, allowed to stand for 15 min, and then scrolled to fully dissolve. The remaining matrix B was divided into Eppendorf (EP) tubes and stored at 4°C for 7 days and –20 °C for 1 month. (Note: when lyophilized powder was opened, the bottle cap was slowly turned to half open, and 5 ml of experimental buffer was added into the crack hole on the side of the bottle cap with 1 ml pipette gun, so as to prevent the powder from flying out.) Ă Preparation of the calibrator: the glass bottle of lyophilized powder of the calibrator was slowly turned to half open to add 250 µl of experimental buffer into the glass bottle. The bottle was slowly turned, so that the lyophilized powder attached to the bottle wall was completely dissolved. The solution concentration was 10,000 pg/ml, marked as C7, and left for 15 min. Before use, the pipette gun was used to draw the solution and rinse the bottle wall several times.
(Note: when the lyophilized powder was opened, the bottle cap was slowly turned to half open, and 125 µl of experimental buffer was added into the two pores on the side of the bottle cap, respectively, to prevent powder from flying out.) Six EP tubes were prepared and labelled as C6, C5, C4, C3, C2, and C1. 75 µl of the experimental buffer solution was added to each tube and then it was diluted step by step by 4 times. That is, 25 µl of C7 solution was taken into six EP tubes, which were gently blown and mixed for 30–40 times to obtain the C6 calibrator with a concentration of 2,500 pg/ml. As with the above method, the calibrators C5, C4, C3, C2, and C1 were diluted by 4 times step by step, respectively. C0 was the experimental buffer.
Steps for sample addition was presented in Table 1.
Table 1
ELISA sample addition procedure
Statistical analysis
Excel 2016 was applied to record and summarize the data, while SPSS 20.0 was used for data statistics and analysis. Mean ± standard deviation (X ± SD) denoted the measurement data, as t test was adopted. Percentage (%) was the representation of enumeration data, which were tested by χ2 test. P < 0.05 was to indicate the statistical differences.
Results
General characteristics of patients
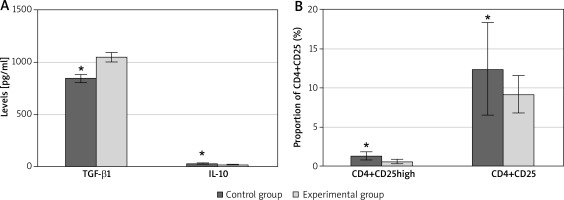
There were male children of 37 (46.25%) cases and female children of 43 (53.75%) cases in the EG, aged 5.7 ±1.4 years old on average, with an average duration of disease of 28.6 ±6.3 months. In the CG, male children were counted as 18 (45%) cases, while female children 22 (55%) cases, with an average age of 5.2 ±1.8 years old (Table 2). Serological tests at the time of treatment showed that in the EG, the serum IL-10 level was 21.4 ±2.8 pg/ml, TGF-β1 level was 1045.7 ±44.7 pg/ml, CD4+CD25 cells accounted for 9.2 ±2.4% and CD4+CD25high cells accounted for 0.6 ±0.3% of CD4+T cells. In the CG, the serum IL-10 level was 34.5 ±3.3 pg/ml, TGF-β1 level was 844.3 ±40.9 pg/ml, CD4+CD25 cells accounted for 12.4 ±5.9% and CD4+CD25high cells accounted for 1.3 ±0.5% of CD4+T cells. Comparing the two groups, the serum level of TGF-β1 in the EG was significantly higher than that in normal children, the level of IL-10 and the proportions of CD4+CD25 cells and CD4+CD25high cells in CD4+T cells were significantly lower than those in normal children. The differences were statistically significant (p < 0.05), as presented in Figure 1.
Comparison of IL-10 and TGF-β1 between groups at discharge
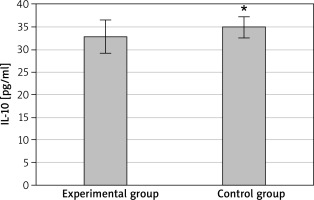
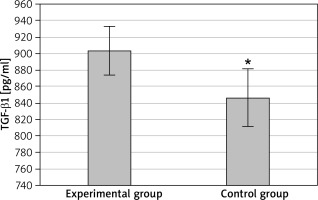
At discharge, the serum levels of IL-10 and TGF-β1 in the EG were 32.8 ±3.7 pg/ml and 903.7 ±29.4 pg/ml, respectively; IL-10 and TGF-β1 levels in the CG were determined as 34.9 ±2.4 pg/ml and 846.3± 35.1 pg/ml, respectively. The serum IL-10 level was highly lower in the EG than that in the CG, with a difference being statistically significant (p < 0.05) as presented in Figure 2. The serum TGF-β1 level in the EG was distinctly higher than that in the CG, with a difference being statistically significant (p < 0.05) as presented in Figure 3.
Analysis of CD4+CD25 in CD4+T cells in two groups at discharge
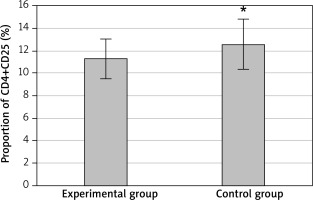
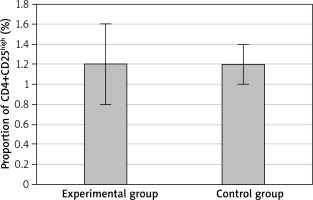
After treatment, CD4+CD25 cells accounted for 11.3 ±1.8% and CD4+CD25high cells accounted for 1.2 ±0.4% of CD4+T cells of the EG. In the CG, CD4+CD25 cells accounted for 12.6 ±2.2% and CD4+CD25high cells accounted for 1.2 ±0.2%. Comparing the two groups, the proportion of CD4+CD25 cells in CD4+T cells in the EG was significantly lower than that in the CG, and the difference was statistically significant (p < 0.05), as shown in Figure 4. There was no significant difference in the proportion of CD4+CD25high cells between the two groups (p > 0.05), as displayed in Figure 5.
Discussion
AA and AR are allergic reactions occurring in different parts of the respiratory tract, with similar causes and pathologic mechanisms. The main clinical manifestations of AR in children include sneezing, a runny nose, nasal congestion, etc., triggered by inhaling cold air, dust, or other allergenic sources. In contrast, the primary clinical manifestations of AA in children involve chest tightness, shortness of breath, dyspnoea, rapid breathing rate, wheezing, etc., following exposure to allergens [14]. Previous studies primarily focused on the Th1/Th2 imbalance theory, with most scholars believing that diseases were caused by an imbalance of Th1/Th2 cells. CD4+T cells differentiated into Th2 cells under allergen stimulation, leading to an increase in cytokines like IL-4 and IL-5. This stimulated B cells to secrete IgE, ultimately resulting in chronic inflammatory responses [15, 16]. However, continuous in-depth research in modern medicine revealed that Th1 cells cannot inhibit Th2-induced inflammation; instead, they exacerbate the disease. The IFN-γ factor secreted by Th1 cells and cytokines produced by Th2 cells fail to cooperate, promoting inflammation and worsening allergic clinical manifestations, including AR and AA [17]. Consequently, this study aimed to observe changes in IL-10, TGF-β1, and CD4+CD25 before and after treatment in children with AA accompanied by AR, providing a reference for the treatment and evaluation of children suffering from AR with AA.
IL-10 has a regulatory effect on AA effector cells and can directly affect inflammatory cytokines. Scholars have reported that IL-10 can reduce the generation of Th cells during immunosuppression of allergic reactions, inhibiting the generation of antigen-presenting cells [18, 19]. Serological results during treatment in the study demonstrated that the serum IL-10 level in the EG was significantly lower than that in normal children. Although it rebounded after treatment, a significant difference persisted. This indicated the involvement of IL-10 in asthma attacks, possibly due to the decreased IL-10 level failing to inhibit Th cell proliferation, resulting in an increase in inflammatory cytokines. After the children received immunotherapy, the increased IL-10 level effectively inhibited the antigen presentation process, induced T cell non-response, and reduced asthmatic manifestations in the children, which is consistent with previous studies [20]. TGF-β1, a type of TGF-β, is a cytokine discovered in recent years, playing a significant role in cellular differentiation, proliferation, and apoptosis. TGF-β1 is a negative immune regulatory factor with pro-inflammatory and anti-inflammatory effects on immune responses and various inflammatory processes [21]. The study showed that the serum TGF-β1 level was notably higher in the EG than in normal children and decreased after treatment, but a significant difference persisted. Similar to previous research findings [22], it was illustrated that the TGF-β1 factor played a role in AR with AA. The increased level of TGF-β1 was associated with inflammatory damage, possibly due to the reconstruction of the airway wall mucosa under the stimulation of inflammatory factors, leading to an increased production of TGF-β1.
CD4+CD25 cells are both important and immunosuppressive. Some scholars have provided evidence that CD4+CD25 Tregs, when stimulated or activated in vitro under a high level of IL-2, do not see a rise in the level of CD4+CD25 cells [23]. For non-T cell receptor-dependent stimulation, the CD4+CD25 cell level does not fluctuate much. However, if the anti-CD28 antibody increases, the CD4+CD25 cell level can be stimulated to recover gradually. CD4+CD25 Tregs can inhibit the transcription and expression of the IL-2α chain gene in target cells, reducing the generation of CD4 and CD8 cells to maintain the body’s immune tolerance [24]. This study showed that the proportion of CD4+CD25 cells and CD4+CD25high cells in CD4+T cells in the EG was significantly lower than that in normal children, with significant differences. However, the proportion of CD4+CD25 cells in CD4+T cells in the EG was still significantly lower than that in the CG. This was consistent with the outcomes of previous relevant research [25]. There are many genetic, biochemical, physiological, and epigenetic findings related to asthma [26–32] and cytokines [33–40]. With the development of desensitization indicators, the percentage of CD4+CD25 cells changed, but that of CD4+CD25high T cells increased compared to that after treatment. Therefore, the CD4+CD25high T cell subset could reduce asthmatic reactions in patients. In the process of immunotherapy, CD4+CD25high T cells gradually recovered to the original level, potentially impacting other cells to achieve the final therapeutic outcome.
Conclusions
In children having AA with AR, the IL-10 cytokine level and the proportion of CD4+CD25 cells among CD4+ cells in peripheral blood were highly decreased, and the TGF-β1 cytokine level was abnormally increased. These might be taken as indicators for treatment or evaluation of patients suffering from AA with AR. However, a large quantity of studies has confirmed that changes in Tregs can be affected by various autoimmune diseases, so the level changes of Tregs alone could not explain its mechanism in the human body. In the future, the effect of Treg/TH17 balance on paediatric AR with AA could be further explored. In conclusion, IL-10, TGF-β1, and CD4+CD25 levels in cells perhaps were of reference value as therapeutic indicators for clinical treatment or evaluation of paediatric AR with AA.