Introduction
Portal vein thrombosis (PVT) is defined as thrombosis that develops in the trunk of the portal vein, including its right and/or left intrahepatic branches. This thrombus may extend to the splenic or superior mesenteric veins [1].
In the pediatric age group, the main incriminated causes of extrahepatic PVT (EHPVT) are direct injury of the portal vein as omphalitis and umbilical vein catheterization (UVC); the incidence of PVT after UVC reaches up to 44% [2, 3]. Other possible causes are neonatal sepsis, exchange transfusion, abdominal or surgical trauma, dehydration, cysts and tumors in the porta hepatis, and cardiovascular malformations. Despite the long list of causes, most of the cases are idiopathic [4, 5].
Acute uncomplicated obstruction of the portal vein mostly presents with sudden onset of ascites, which in most cases resolves spontaneously. A collateral circulation develops in order to bypass the block. After this the patients remain asymptomatic for a period of time until other features of portal hypertension appear. Acute symptoms are often absent in young patients and 80% present for the first time with variceal bleeding. Splenomegaly is a very common feature and sometimes the physical discomfort caused by this large organ is the only significant clinical complaint. Most of the patients have trivial liver biochemical changes; however, hypersplenism is common and often severe enough to cause profound anemia [6].
On ultrasound examination, solid isoechoic or hypoechoic material within the portal vein is visible, either filling the lumen partially or completely in the acute stage of the thrombosis. Once diagnosed, it is important to carry out endoscopy in patients with PVT as large esophageal varices may be present [7].
In chronic PVT gastric varices are seen in nearly 40% of patients. Large varices are an independent risk factor for bleeding in patients with PVT. However, despite the presence of large varices, with red signs, the bleeding frequency in patients with PVT is 0.25% over 2 years, which is far less than in cirrhotics with similar variceal characteristics (20-30% bleeding risk over 2 years) [8].
The treatment of portal venous obstruction depends upon the patient’s age, the site and nature of the obstruction and the clinical features. Treatment of variceal bleeding in children is challenging and with less well-established treatment modalities due to shortage of proper evidence that can be used to assess the risk and benefits of a particular strategy. Endoscopic variceal ligation (EVL) is the primary choice for the management of variceal bleeding in children. This treatment may be technically difficult in young and small children; sclerotherapy is then recommended as an alternative approach in such cases [9].
We aimed in this work to discuss our experience as a developing country in management of children with extra-hepatic PVT hoping to delineate better diagnostic methods and treatment strategies.
Material and methods
The study included 62 children with EHPVT without associated chronic liver disease who were being followed at Dr. Yassin Abdel Ghaffar Charity Center for Liver Disease and Research, which is a tertiary referral center located in Cairo, the capital of Egypt, and which receives patients from rural and urban areas in upper and Lower Egypt. The study included the patients who presented to our center in the period from January 2012 to January 2021. This study was compliant with Declaration of Helsinki guidelines, 2013, and was approved by the Medical Ethics Committee of Ain-Shams University, Egypt. The requirement for informed consent was waived due to the retrospective nature of the study.
The diagnosis of PVT was established by ultrasound and Doppler where obstruction of flow or thrombus and/or cavernomatous transformation of the portal vein was observed. The exclusion of an associated hepatic disease was carried out through clinical, laboratory and radiological evaluation.
The study was a retrospective cohort one. All patients’ records were reviewed, including the entire medical and nursing staff records, the medical prescriptions, laboratory and radiological reports. The patients’ socio-demographic data, post-natal history, disease presentation and clinical examination on first presentation to our center were collected. Laboratory investigations (complete blood count, liver function tests, and renal function tests), abdominal ultrasound/Doppler for liver size, splenic size, presence of thrombus, portal cavernoma, collaterals (diameter and velocity), velocity in right and left portal branches, biliary passages dilatation, gallbladder stones, upper endoscopic findings and treatment regimens on initial disease presentation and during the regular follow-up visits were collected whenever available.
A wide range of potential risk factors of PVT were selected to be evaluated such as infections in the perinatal period, NICU admission and use of an umbilical catheter. Assessment of hypercoagulability was available in some cases.
Statistical methods
Data were collected and analyzed using SPSS, version 18.0 (SPSS Inc., Chicago, Illinois, USA). Qualitative data were expressed as frequency and percentage (%). Quantitative data were shown as mean ± standard deviation (SD) or median as appropriate. Significance was tested by Fisher’s exact test for qualitative variables. Student’s t-test or the Mann-Whitney U test was used to compare 2 sets of quantitative variables according to the nature of the data. The p (probability) value was considered significant if it was < 0.05.
Results
Sixty-two children with EHPVT, without associated liver disease, were assessed. 62.9% of patients were male and 37.1% were female. The age at disease presentation ranged from 3 months to 13.5 years, with a mean age of 3.5 ±2.7 years (median: 3 years). The patients presented to us at our center at mean age of 5.07 ±3.2 years (range: 1.2-14 years, median: 4 years). Thirty-two percent of cases were born to consanguineous couples. Thirty-five percent were born through normal vaginal delivery and 65.2% by caesarian section; 63.3% were full term while 36.7% were pre-term.
Seventy-eight percent of cases were admitted to the NICU after birth. The mean duration of NICU admission was 34.2 ±18.9 days (range: 5-75 days). History of umbilical catheterization was present in 60% of cases and neonatal sepsis was present in 21.4%. One fourth of the patients (25.5%) received blood transfusion during the neonatal period. History of dehydration was present in 1.6%. Seven patients (11.3%) had undergone abdominal surgery: herniotomy in 6 cases and 1 patient underwent multiple intestinal operations. Thirteen percent of the patients had no risk factors.
The main initial clinical presentation of the disease was hematemesis and/or melena (30 cases; 48.4%). The median age for presentation with hematemesis and/or melena was 3 years, with range from 6 months to 13.5 years. The precipitating factors of the bleeding were receiving non-steroidal anti-inflammatory drugs (NSAID) in 10 cases (33.3%) while in the remaining cases the precipitating factor was not clear (66.7%).
Nearly half of the patients (45.2%) were accidently discovered to have splenomegaly while seeking medical advice for another disease. Their median age was 2.5 years (range: 3 months to 11 years). Two cases presented with abdominal enlargement at ages of 1.5 and 2 years and another 2 presented with pallor at the ages of 1.9 and 5 years.
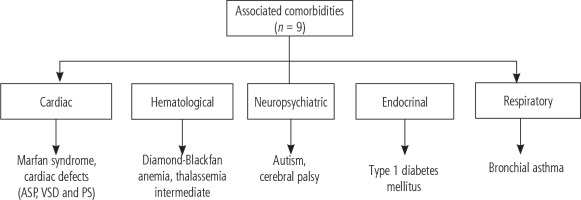
Nine cases had associated comorbidities: autism, cerebral palsy, Marfan syndrome, cardiac defects (atrial septal defect [ASD], ventricular septal defect [VSD] and pulmonary stenosis [PS]), type 1 diabetes mellitus, Diamond-Blackfan anemia, thalassemia intermedia, bronchial asthma, and multiple congenital anomalies – one case each (Fig. 1).
During the clinical examination carried out at the first presentation of the patients to us, the weight of 23.5% and the height of 15.4% of the patients were below the 3rd percentile. Hepatomegaly was present in 38.3% of cases while splenomegaly was present in 91.7% of cases. Jaundice, ascites, petechial rash and ecchymosis were not observed in any of the patients on initial examination.
Tests of hypercoagulability were done for 17 patients; in 12 of them (70.6%) coagulation disorders were present. Eight patients had an isolated deficiency (4 had isolated anti-thrombin III deficiency, 3 had protein C deficiency, and one had an isolated factor II mutation). Four patients had combined deficiencies (2 suffered combined protein C, S and antithrombin-III deficiency, 1 had combined protein C and S deficiency, and 1 had combined anti-thrombin III and MTHFR deficiency). Half of the 12 patients with coagulation disorders also had a history of local causes of thrombosis (4 had a history of umbilical catheterization, 1 had a history of neonatal sepsis, 1 case had undergone multiple intestinal operations). Also 3 patients had a history of packed red blood cells (RBC) and plasma transfusion and 1 patient suffered from Diamond-Blackfan anemia. No family history of PVT was recorded in any of the patients.
Laboratory assessment during the first visit showed that 43.1% of patients had elevated hepatic enzymes (aspartate aminotransferase and/or alanine aminotransferase); most of them (85.7%) had only one-fold elevation, 9.5% had 2-fold elevation and 4.8% had 3-fold elevation. Around one fifth of the patients (22.2%) had hypoalbuminemia. Hematological abnormalities were present in the majority of the cases; 20% had pancytopenia and 40% had bicytopenia. Isolated thrombocytopenia and anemia were present in 12.7% and 23.6% respectively. Only 12.7% had normal complete blood count (CBC) parameters. The other laboratory parameters are shown in Table 1.
Table 1
Clinical and laboratory parameters of patients with extra-hepatic portal vein thrombosis (EHPVT) at disease presentation
Ultrasound/Doppler evaluation revealed presence of hepatomegaly in 55.3% and splenomegaly in 89.6% of the patients. Presence of collaterals was noted in 76.2%; short gastric vessels and lienorenal collaterals were present in 58.3% and 63.6% of patients, respectively (Table 2). Gall stones were present in 2 cases. Biliary dilatation and evidence of portal biliopathy appeared in one case.
Table 2
Ultrasound evaluation of patients with extra-hepatic portal vein thrombosis (EHPVT) at disease presentation
Patients who presented with hematemesis and/or melena were compared to patients who came with other presentations; patients’ gender, consanguinity, family history, type of delivery, gestational age, history of NICU admission and umbilical catheterization, presence of hepatomegaly and splenomegaly, and Doppler findings did not differ significantly between groups (p > 0.05), while the patients’ age at presentation to us was significantly higher in patients who presented with hematemesis and/or melena (p < 0.05). In addition, the mean serum albumin level was significantly lower in patients who presented with hematemesis and/or melena (p < 0.05) (Table 3).
Table 3
Comparison between patients who presented with hematemesis and/or melena (n = 30) and patients who presented with other conditions (n = 32) on first presentation to us
Table 4
Endoscopic findings of patients with extra-hepatic portal vein thrombosis (EHPVT) on disease presentation
All patients who presented with hematemesis/melena or had a history of either of them were endoscopied. Varices were managed either by band ligation or injection sclerotherapy (according to age). These patients were put on propranolol for secondary prophylaxis. Patients who did not present with hematemesis/melena were assessed by ultrasound/Doppler. If splenomegaly was present and/or platelet count was less than 150, diagnostic endoscopy was done.
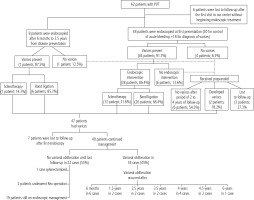
Out of the 62 patients, 48 had undergone upper endoscopy (30 with hematemesis/melena+ 18 without hematemesis/melena) on first presentation to us; 44 of them were found to have varices and 38 needed endoscopic intervention. In the other 4 cases there were no varices. At patients’ followup, another 8 cases had undergone their first endoscopy 6 months to 3.5 years after their first presentation (7 cases had varices and 1 case had no varices). Six patients were lost to followup without doing even the first diagnostic endoscopy. Figure 2 shows the follow-up of the patients. The median time for first obliteration of varices was 2.5 years. Endoscopic intervention was done as management of acute bleeding, as primary prophylaxis in 33.3% of cases and as secondary prophylaxis in 66.7%.
The 5 patients who had no varices and the 6 patients who had low grade varices received propranolol as primary prophylaxis after their first endoscopy. At follow-up, 6 patients did not develop varices and stayed on medical treatment during the follow-up period (from 2 to 4 years), only 2 needed endoscopic intervention with band ligation and 3 cases were lost to follow-up after the first endoscopy (Fig. 2).
On comparing patients with and without varices on first presentation of the disease, the distribution of the patients’ gender, age, consanguinity, family history, type of delivery, gestational age, and history of NICU admission or umbilical catheterization was comparable in both groups (p > 0.05), except for the mode of presentation; 59.1% of patients with varices presented with hematemesis and/or melena, while 75% of patients without varices were accidently discovered (p = 0.033). In addition, clinical examination showed no significant difference between the groups (p > 0.05).
The outcome of management of varices with band ligation and injection sclerotherapy was nearly equal, with no statistically significant difference (p > 0.05).
Splenectomy was done in only one case due to presence of huge splenomegaly disabling movement and 2 cases underwent the Rex operation due to rapid recurrence of varices and frequent attacks of hematemesis despite regular endoscopic management. Complications of liver failure were not reported in any of the cases. None of the cases developed hepatopulmonary syndrome. No one developed re-thrombosis during the follow-up period.
Discussion
Portal vein thrombosis is a common cause of portal hypertension. To date, the pathogenesis of PVT in children still remains unexplained, yet it is the major cause of portal hypertension in children and adolescents. Variceal bleeding due to PVT is a recognized cause of upper gastrointestinal bleeding in children in developing countries [10].
The most common clinical presentation in our patients was hematemesis and/or melena (48.4%), which was triggered by the use of NSAIDs in nearly one third of cases. In fact, in most studies, upper gastrointestinal bleeding was the main clinical presentation in children with EHPVT (over 50%) [11]. According to other authors, about 79% of children with EHPVT will develop at least one episode of upper gastrointestinal bleeding during their lifetime [12].
The median age for presentation with hematemesis and/or melena in our cohort was 3 years. The manifestations of EHPVT are usually absent during the neonatal period and appear later in childhood [13]. However, in older children and teenagers, the risk of variceal bleeding decreases due to the development of spontaneous portosystemic collaterals [11].
The second most frequent clinical presentation of EHPVT in our children was accidently discovered splenomegaly (45.2%), with its consequences (pancytopenia, bicytopenia or isolated thrombocytopenia secondary to hypersplenism).
Most of our patients had a normal growth pattern, as the PVT did not affect the entire liver function. Linear growth retardation was encountered in nearly 15% of our children. Intestinal malabsorption secondary to venous stasis, anorexia secondary to splenomegaly, or fear of bleeding may be contributing factors for growth retardation. Moreover, growth retardation in EHPVT may be secondary to resistance to growth hormone action and reduction of hepatotrophic hormone synthesis due to decreased hepatic vascularization [13, 14].
For the EHPVT diagnosis, we used abdominal Doppler ultrasound examination as the method of choice. Presence of splenomegaly and cavernous transformation with collateral circulation was a prominent finding in most of the patients.
In the current study, the majority of patients with pediatric PVT had a history of a local prothrombotic factor, such as UVC (60% of cases) or sepsis (21.4%). Therefore, and distinctly from adult PVT, local factors seem to be the major players implicated in the development of PVT in children. In a study from Toronto, Canada, among 133 neonates who underwent UVC, 72.93% developed EHPVT [15, 16]. Furthermore, UVC was the main etiological factor of EHPVT (65%) in a group of 187 Italian children, 61% of them being premature [17]. In Brazil, a history of UVC was present in 40.6% of children with EHPVT [18].
Other factors such as blood transfusion, abdominal surgery, hereditary thrombophilias and dehydration also have been suggested to play a role in PVT development.
Hereditary thrombophilias that include certain mutations of the prothrombin (PTHR), factor V Leiden (FVL) or methylenetetrahydrofolate reductase (MTHFR) genes or deficiency of one of the natural anticoagulant proteins C and S are known to predispose to venous thrombosis mainly if they are associated with local factors [19]. Abnormal values of the anticoagulation proteins were observed in a significant number of patients who were tested for thrombophilia disorders (70.6% of the investigated patients had isolated or combined inherited coagulation disorders). In Grama et al.’s study, the frequency of protein C, protein S, or antithrombin III deficiency was estimated at 40-50% of children with EHPVT [11].
Protein C or S deficiency may be associated with EHPVT in children and adolescents without being proved to be the determining factor. The decreased level of these proteins can be secondary to the thrombosis itself, or can result from their consumption in portosystemic shunts. The neovascular formations (cavernoma) will cause a hepatopetal flow that is not sufficient to reduce the pressure, and spontaneous natural portosystemic shunts may be formed. These shunts function as “release valves” to reduce the pressure in the portal space. But this compensatory mechanism is insufficient and does not allow adequate reduction of portal pressure, thus causing the consumption of these anticoagulant proteins. Another cause of protein C or S deficiency is the hepatic injury caused by reduced flow through the portal vein [20].
To date, there is no universally accepted follow-up and treatment protocol for children with EHPVT. Our treatment goals were emergency measures to stop variceal bleeding and prevent the first episode of bleeding (primary prophylaxis) and recurrent variceal bleeding (secondary prophylaxis). Treatment included medical (b-blockers) or endoscopic measures (sclerotherapy and variceal band ligation).
Sclerotherapy and variceal band ligation are essential treatment methods both in severe life-threatening bleeding and in preventing potential bleeding in those with high-grade varices. Nearly 87% of our children needed intervention with either variceal band ligation or sclerotherapy, the latter being used mainly in young children where introduction of banding instruments may be injurious. Endoscopic management with sclerotherapy and variceal band ligation in conjunction with medical management with β-blockers were effective in 45% of patients with large varices, with a median time for first obliteration of 2.5 years.
Eleven patients with no or low grade varices received medical management only with propranolol as primary prophylaxis for variceal bleeding with a good response (no bleeding) over a follow-up period of 2-4 years in nearly half of the cases. A study from Turkey on the usefulness of β-blocker therapy revealed that, out of 45 children with EHPVT treated with propranolol, 8 children (15.6%) presented with upper gastrointestinal bleeding during primary prophylaxis [21]. Most studies report a bleeding rate among those who received treatment with propranolol between 2% and 11% per year of follow-up [22]. According to other authors, the degree of varices decreased with β-blocker therapy, but the results do not reflect the usefulness of their use in children [23].
Hence, the effectiveness of β-blockers for the prevention of esophageal varices in children with EHPVT remains unclear. The decision to administer or not depends on the experience of each center. In our center, we used propranolol treatment in a significant number of cases; it was effective when used as primary prophylaxis, while its effect as a secondary prophylaxis was difficult to interpret.
As a result of the effective management of esophageal varices in children with endoscopic banding or sclerotherapy, the number of referrals for surgery has decreased remarkably in recent years. However, progressive hypersplenism may occur even after successful sclerotherapy [24]. Surgery was indicated in 3 of our patients. One child was splenectomized due to presence of huge splenomegaly disabling movement with significant thrombocytopenia. Two children with recurrent bleeding despite regular endoscopic management underwent the Rex operation with a favorable outcome and eradication of varices after the surgery.
Meso-Rex bypass aims to restore the portal vein’s flow by creating an anastomosis between the superior mesenteric vein and the left portal vein. The meso-Rex shunt reduces the portal vein pressure, the degree of esophageal and gastric varices, and the splenomegaly, and significantly improves the prognosis in children with EHPVT [25].
In Grama et al.’s study, surgical treatment was applied in 15 children (23.8%) due to recurrent gastrointestinal bleeding and/or severe thrombocytopenia. Distal splenorenal shunt was performed in 6 patients (9.5%), mesorenal shunt in 2 patients (3.2%), and mesocaval shunt in 3 patients (4.8%). Meso-Rex bypass was successfully performed in 4 children (6.3%). Five of the 15 patients who received surgical treatment suffered complications (thrombosis); 2 of them had thrombophilia disorders [11].
From the previous results we recommend that Doppler ultrasonography should be a routine investigation for neonates admitted to the NICU before discharge, especially those exposed to UVC or neonatal sepsis. Investigation for thrombophilia disorders should be done for any patient at risk for PVT as who has been exposed to abdominal surgery in order to delineate further followup strategies.
Conclusions
In our cohort, variceal bleeding was the most common clinical presentation of EHPVT in children. UVC is still the main etiological factor of EHPVT in our cohort, especially in the presence of a thrombophilic disorder. Endoscopic management (band ligation or sclerotherapy) was effective in attaining variceal obliteration in nearly half of the patients.