Summary
Extracellular vesicles promote in vivo angiogenesis in myocardial infarction rats via the miR-423-5p/EFNA3 axis, thus improving cardiac repair.
Introduction
Myocardial infarction (MI), as a predominant cause of cardiovascular disease (CVD) mortality [1], is a devastating condition that occurs when coronary arteries are blocked and regional blood supply to the myocardium is impaired, resulting in cardiomyocyte death [2]. This disease is lethal to patients due to insufficient blood perfusion to important organs [3]. MI is a severe disease that has an association with angiogenesis dysfunction, and cell-based treatment approaches for MI with mesenchymal stem cell (MSC)-secreted exosomes have been investigated owing to their strong proangiogenic effect [4]. Therefore, it is vital to investigate the underlying molecular mechanism related to MI in treating the disease.
MSCs are able to simultaneously activate several mechanisms, and MSC transplantation is believed to be the best and most efficient method in cell therapy [5]. Human MSCs attract particular interest as a common and feasible add-on treatment for MI [2]. Human umbilical cord mesenchymal stem cells (hucMSCs) have strong self-renewal ability and multi-potency, and offer possibilities of replacing injured cardiomyocytes [6]. Extracellular vesicles (EVs) are a heterogeneous group of membrane-limited vesicles loaded with various proteins, lipids, and nucleic acids [7]. As small membrane-bound particles produced from cells, it is indicated that EVs possess cardioprotective effects [8]. It is reported that EVs can powerfully promote angiogenesis and guard the heart against MI. In addition, EVs derived from different sources might become helpful biomarkers of cardiovascular diseases [9]. The intercellular communications between MSCs and cancer cells through MSC-EV-microRNA (miRNA) have suggested that modification of hucMSC-derived EVs (hucMSC-EVs) may be an attractive treatment selection for hucMSC-EVs’ clinical application [10].
miRNA have attracted considerable attention for the capability to orchestrate changes to the transcriptome, and finally the proteome, in heart failure [11]. Evidence has shown that miR-423-5p possesses a cardiac origin [12], and miR-423-5p is associated with congestive heart failure [13]. Moreover, miR-423-5p might act as valuable biomarkers in patients with left ventricular (LV) heart failure or patients with LV remodeling after MI [14]. EphrinA3 (EFNA3) is one member of the ephrin protein family that elicits short-distance cell-cell signaling, and it promotes or represses tumorigenesis depending on tumor types [15]. Specifically, EFNA3 is implicated in vascular remodeling and apoptosis in the heart [16]. Besnier et al. found that in endothelial cells, miR-210 represses EFNA3, inducing proangiogenic responses [17].
Aim
Taking the active functions of EVs, miR-423-5p, and EFNA3 into consideration, we performed this study to discover the essential role of hucMSC-EVs’ delivery of miR-423-5p in MI by targeting EFNA3.
Material and methods
Ethics statement
The use of all animals was ratified by the Experimental Ethics Committee of our hospital.
Cell culture
HucMSCs and human umbilical vein endothelial cells (HUVECs), procured from Wuhan Procell Life Science & Technology Co., Ltd. (Wuhan, China), were incubated in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. These cells were passaged every 3 days, and the hucMSCs at passages 3-7 were utilized for subsequent experiments [18].
HucMSC identification
Multiplex differentiation ability: HucMSCs were incubated in OriCell hucMSC osteogenic and adipogenic differentiation medium (Cyagen Biosciences Inc., Guangzhou, China), respectively, and stained using oil red O solution and alizarin red solution, respectively, to evaluate the accumulation of intracellular lipid droplets and calcium deposits [19, 20].
EV isolation and identification
EVs were extracted in vitro from the hucMSC medium. Before utilization, FBS in the medium was ultracentrifuged at 100,000 g for 12 h to remove EVs from the serum. The cells were inoculated in 6-well plates at 2 × 105 cells/well, and continued to incubate with an EV-free serum for 48 h for the harvest of the cell supernatants. Subsequently, hucMSC-derived EVs (hucMSC-EVs) were isolated by differential centrifugation. All the centrifugation steps were done at 4°C, while the other steps were conducted on ice. The specific steps were as follows: centrifugation at 500 g for 15 min, 2000 g for 15 min, and 10,000 g for 20 min to remove dead cells and debris. A 0.22 µM filter was utilized to remove smaller cell debris and then ultracentrifuged at 100,000 g for 70 min, followed by resuspension in phosphate-buffered saline (PBS) for a second ultracentrifugation under the same conditions. The precipitate was stored at –80°C for further or immediate use.
EV identification with transmission electron microscopy (TEM): 20 µl of EVs was supplemented dropwise on the copper grid, left for 3 min, and then supplemented dropwise with 30 µl of phosphotungstic acid solution (pH 6.8) after aspirating the liquid from the side with filter paper, re-stained for 5 min at room temperature, then dried with incandescent light, and photographed under the TEM.
EV surface markers were identified by western blot: EV suspensions were concentrated and their protein content was determined by a BCA kit (Thermo Fisher Scientific, MA, USA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels were prepared, and then protein denaturation and electrophoresis were performed. After that, membranes were transferred and assayed for EV-specific marker proteins Hsp70 (ab5442), CD81 (ab79559), Alix (ab117600), and Calnexin (ab112995) (all from Abcam, UK) [21, 22].
Uptake of hucMSC-EVs by HUVECs
EVs were marked with the PKH26 Red Fluorescent Kit (Sigma Aldrich, USA). Specifically, EVs collected after 100,000 g ultracentrifugation were cultured with PKH26 dye previously diluted in diluent C solution at room temperature for 10 min. The labeled EVs were rinsed by PBS ultracentrifugation. PKH26-labeled EVs were suspended in a low-serum medium and cultured with HUVECs for 12 h. Cell nuclei were stained with 4′-6-diamidino-2-phenylindole (DAPI) and analyzed by fluorescence microscopy.
Lentiviral and plasmid packaging, and cell transfection
For lentiviral infection, hucMSCs during the logarithmic growth phase were digested with trypsin, counted, and then inoculated in 6-well plates with DMEM diluted for transfection. The lentiviral plasmid encoding miR-423-5p and its corresponding negative control (miR-NC) were designed and produced by Genechem (Shanghai, China). HucMSCs were transfected with the lentivirus at a multiplicity of infection (MOI) of 20. These cells were screened with 1 µg/ml of puromycin for 3 days.
HucMSC grouping and treatment: cultured hucMSCs were separated into the PBS group, EV group (extraction of hucMSC-EVs), EV-miR-NC group (extraction of hucMSC-EVs containing the lentiviral plasmid miR-NC), and EV-miR-423-5p group (extraction of hucMSC-EVs containing the lentiviral plasmid miR-423-5p).
HUVECs were transiently transfected. pcDNA-3.1 EFNA3, miR-423-5p mimic, and the corresponding NCs were obtained from Genechem. HUVECs were cultured in 6-well plates at an inoculation density of 4 × 105 cells/ml. When cell fusion reached 80%, the cells were transfected following Lipofectamine 2000 instructions (Invitrogen, New York, California, USA). Next, the cells were transfected with 250 µl of serum-free Opti-MEM (Gibco) to dilute miRNA mimic or plasmid in each group and evenly mixed with 10 µl of Lipofectamine 2000 for 5 min, and supplemented dropwise to the cell culture wells after standing for 20 min. After transfection, the cells of different groups were incubated at 37°C under 5% CO2 for 48 h for subsequent related experiments.
Co-culture experiments
HUVECs were inoculated in 6-well plates, and after reaching 70-80% confluence, they were incubated with PBS and EVs, respectively, for 24 h, and then incubated with hypoxia and serum deprivation (h/SD) for 12 h for subsequent experiments. The researchers had no knowledge of the group assignments during the experiments [18].
HUVECs angiogenesis test
The capillary tube formation assay was utilized to assess HUVECs’ angiogenesis. HUVECs (30,000 cells/well) were inoculated into 96-well plates covered with growth factor-reduced Matrigel. Capillary-like tube formation was photographed 6 h later. Image J software (National Institutes of Health, NIH) was utilized to quantify tube length.
Transwell assay
In vitro cell migration assays were conducted in 24-well plates using a Transwell chamber (pore size: 8 mm, Corning, USA). A Transwell chamber without Matrigel was prepared, and the lower chamber was prefilled with 600 ml of 20% FBS medium and equilibrated at 37°C for 1 h. HUVECs were resuspended in an FBS-free medium, and 1 × 106 cells were inoculated into the upper chamber and incubated at 37°C and 5% CO2 for 24 h. After that, the Transwell chamber was fixed with 5% glutaraldehyde at 4°C, and dyed with 0.1% crystal violet for 5 min. The surface cells were wiped off with cotton balls, observed under an inverted fluorescence microscope (TE2000, Nikon, China), and photographed from 5 randomly selected fields. The number of cells through the chamber per group was regarded as the average value. Differences between groups were analyzed and histograms of migration were plotted.
Enzyme-linked immunosorbent assay (ELISA)
VEGF concentration in serum and cell supernatants was tested with an ELISA kit (MSKBIO, Wuhan, China). The absorbance (A) values of each well at 450 nm were tested within 3 min using a full-range microplate reader (Synergy 2, BioTek, USA). To calculate the regression equation of the standard curve, the standard concentration was utilized as the horizontal coordinate and the A value as the vertical coordinate, and the sample A value was substituted into the equation to calculate the concentration of the target protein in the sample [23].
Luciferase assay
Bioinformatics software (http://www.targetscan.org) predicted the targeting relationship between miR-423-5p and EFNA3 and the binding site of miR-423-5p to the EFNA3 3′UTR. The 3′-UTR sequence of EFNA3 containing the presumed miR-423-5p binding sites (WT) or mutated binding sites (MUT) were inserted into the pGL3 (Promega, WI, USA) plasmid, thereby constructing the EFNA3-WT and EFNA3-MUT plasmids. Logarithmically grown HUVECs were inoculated in 96-well plates and then transfected with Lipofectamine 2000 (Invitrogen). EFNA3-WT and EFNA3-MUT, together with miR-423-5p mimic and its NC, were transfected into HUVECs with 3 replicates per group. After 48 h, the Dual-Luciferase Reporter Assay System kit (Promega, USA) was implemented to detect luciferase activity [24].
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was extracted by the TRIzol method (Invitrogen). miRNA was reversely transcribed utilizing the MiRcute miRNA First-strand cDNA synthesis kit (Tiangen Biotech, Beijing, China) and mRNA was reverse-transcribed implementing the PrimeScript RT reagent Kit (Promega, Madison, WI, USA). After that, SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA, USA) was used to quantify gene expression. The relative transcript levels of the target genes were determined using the relative quantification method (2–ΔΔCT method). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was considered as the internal reference gene for mRNA and U6 was considered as the internal reference for miRNA. All reactions were repeated 3 times. The primer sequences are detailed in Table I.
Table I
Primer sequences for RT-qPCR
MI animal model establishment
Male Sprague Dawley rats (6–8 weeks old) were housed in standard rat cages with ventilation, quietness, and natural light. The room temperature was maintained at 23–25°C; the relative humidity was kept at 55–60%. The rats were fed and watered freely, and the bedding in the cages was changed daily by a dedicated person.
The rats were anesthetized intraperitoneally with pentobarbital sodium (50 mg/kg) and then connected to a ventilator via oral tracheal intubation. After fixation, a left-sided thoracotomy was conducted between the 3rd and 4th intercostal space under aseptic conditions, and the anterior descending branch of the left coronary artery was ligated 1.5 mm below the inferior border of the left auricle. After the MI model was successfully established, MI rats were randomly grouped into 4 groups (n = 10): 1) MI group: the MI model was established; 2) MI + EV group: after successful establishment of the MI model, EVs (50 µg) were injected at the border of the infarct area; 3): MI + EV-miR-NC group: after successful establishment of the MI model, EV-miR-NC (50 µg) was injected at the border of the infarct area; 4): MI + EV-miR-423-5p group: after successful establishment of the MI model, EV-miR-423-5p (50 µg) was injected at the border of the infarct area. The sham group was regarded as the control group (n = 10). Carprofen (10 mg/kg) was utilized for postoperative analgesia to ensure that the animals did not suffer additional pain. All those who performed surgery and subsequent analyses were blinded to the intervention [18, 25].
Cardiac function evaluation
Cardiac function was assessed by transthoracic echocardiography (VEVO 2100, VisualSonics, USA) after 2 weeks of EV treatment. The rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg) and put on a heated platform in the supine position. The LV internal diameter was determined by a 30 MHz transducer from a short-axis view recorded in m-mode. Left ventricular ejection fraction (LVEF) and left ventricular shortening fraction (LVFS) were recorded by implementing Vevo analysis software to evaluate cardiac function.
Masson staining
To detect fibrosis, heart tissue sections embedded in paraffin were stained with Masson staining [26]. The infarct area was measured as the average ratio of fibrotic area to total ventricular area. The sections were observed and photographed under a microscope, and analyzed by Image J software.
Immunohistochemistry
Paraffin sections of heart tissues were heated at 60°C for 1 h, dewaxed and hydrated in xylene I and xylene II, dehydrated in gradient alcohol, soaked in 3% hydrogen peroxide at room temperature for 20 min to eliminate endogenous peroxidase activity, and blocked with 10% goat serum for 15 min. Primary antibodies CD31 (ab28364, 1 : 50, Abcam, USA) and VEGF (19003-1-AP, 1 : 200, Proteintech, Rosemont, IL, USA) were added to the sections and cultured overnight at 4°C, then biotin-labeled goat anti-rabbit IgG (ab6721, Abcam) secondary antibody working solution was added dropwise and cultured for 40 min at room temperature. After that, the sections were developed by DAB for 10 min, re-stained with hematoxylin for 1 min, dried in gradient alcohol dehydration, cleared by xylene, and blocked utilizing neutral gum. PBS was used as an NC instead of a primary antibody. The final results were double-blindly scored by two persons [27].
Statistical analysis
The experiment was repeated at least 3 times. SPSS 21.0 software and GraphPad Prism 8.0 software were implemented for assessing statistical differences. The data were expressed as mean ± standard deviation. Comparison of data between two groups was performed by the unpaired t-test, and one-way analysis of variance (ANOVA) was implemented for comparison among multiple groups, with Tukey’s post hoc test. *P < 0.05 was considered to indicate statistical significance.
Results
hucMSC-EVs are enriched with miR-423-5p
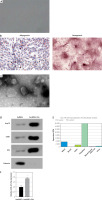
To investigate the role of EVs secreted by hucMSCs, we first identified hucMSCs. Under light microscopy, hucMSCs were long spindle-shaped or spindle-shaped, growing in colonies, and the cells were arranged in a swirling pattern when densely packed (Figure 1 A). Meanwhile, hucMSCs were subjected to adipogenic and osteogenic differentiation assays, and it was found that hucMSCs had good adipogenic and osteogenic differentiation (Figure 1 B). EVs were further isolated from hucMSCs. TEM analysis revealed that EVs isolated from hucMSC supernatant were cup-shaped (Figure 1 C). Western blot results (Figure 1 D) showed that the hucMSC-EVs expressed characteristic proteins such as CD81, Alix, and Hsp70, while they negatively expressed calnexin. These results indicated that we successfully isolated hucMSC-EVs. We found miR-423-5p presented in MSCs by the EVmiRNA Database (Figure 1 E), and we further examined the content of miR-423-5p in hucMSC-EVs using RT-qPCR, and found that miR-423-5p could be enriched in hucMSC-EVs (Figure 1 F).
Figure 1
HucMSC-EV identification. A – HucMSCs P3 morphology was observed under a light microscope; B – Oil red O and alizarin red stainings were utilized to detect adipogenic and osteogenic differentiation of hucMSCs, respectively; C – EV morphology was observed by transmission electron microscopy D – Expression of positive and negative characteristic markers of hucMSC-EVs was determined by Western blot; E – EVmiRNA database (http://bioinfo.life.hust.edu.cn/EVmiRNA#!/) was applied to detect miR-423-5p distribution in EVs from diverse sources; F – miR-423-5p content in hucMSC-EVs was measured by RT-qPCR. *P < 0.05 vs hucMSC group
The above findings suggested that hucMSC-EVs were enriched with miR-423-5p, and miR-423-5p was delivered to HUVECs.
hucMSC-EVs promote in vitro tube formation of HUVECs by delivering miR-423-5p
To further explore whether hucMSC-EVs could enter into HUVECs, we co-incubated PKH26-labelled EVs with HUVECs. Fluorescence microscopy (Figure 2 A) revealed that PKH26-labelled hucMSC-EVs were gradually internalized by HUVECs. miR-423-5p expression in hucMSCs and hucMSC-EVs was further tested by RT-qPCR, and the experimental results demonstrated that miR-423-5p expression was increased in both hucMSCs and EVs infected with miR-423-5p (Figure 2 B). The above results indicated that HUVECs could take up hucMSC-EVs, thereby increasing miR-423-5p expression levels in HUVECs.
Figure 2
HucMSC-EVs promote in vitro tube formation of HUVECs by delivering miR-423-5p. A – HUVECs were cultured with PKH26-labelled EVs (red) and endocytosis of EVs was detected by immunofluorescence assay; B – miR-423-5p expression in hucMSCs and EVs was tested by RT-qPCR; C – miR-423-5p expression in HUVECs was measured by RT-qPCR; D – VEGF content in cell supernatants was measured by ELISA. E – Angiogenic ability of HUVECs in each group was evaluated by pseudo-tube formation assay. F – Migration ability of HUVECs in each group was detected by Transwell assay.*P < 0.05 vs. PBS group or miR-NC group, #p < 0.05 vs. EV-miR-NC group
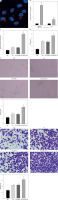
To determine whether miR-423-5p transferred in vitro could effectively regulate HUVECs’ angiogenic capacity, we overexpressed miR-423-5p in hucMSCs, then isolated EVs and co-cultured EVs with HUVECs, and the results indicated that miR-423-5p expression was elevated in HUVECs after co-culture with EVs or EV-miR-NC, and miR-423-5p expression was higher in HUVECs co-cultured with EV-miR-423-5p (Figure 2 C). The migration and angiogenic abilities of HUVECs after co-culture with hucMSC-EVs were further examined, and the results revealed that HUVECs’ migration and angiogenic ability and VEGF levels were raised upon treatment with EVs, while the increase in migration and angiogenic capacity of HUVECs, as well as VEGF levels, upon treatment with EV-miR-423-5p was more significant (Figures 2 D–F).
The above experimental results demonstrated that hucMSC-EVs could promote in vitro tube formation by delivering miR-423-5p to HUVECs.
miR-423-5p inhibits EFNA3
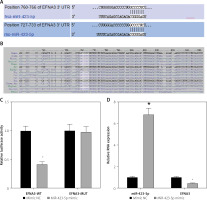
Previous studies have demonstrated that EFNA3 has a significant association with angiogenesis and that EFNA3 downregulation promotes angiogenesis [17, 28]. The bioinformatics website TargetScan predicted that miR-423-5p and EFNA3 had specific binding regions and were highly conserved in multiple species (Figures 3 A, B). Luciferase assay was further conducted to verify that EFNA3 was a target of miR-423-5p. EFNA3-WT luciferase activity was reduced in hucMSCs transfected with miR-423-5p mimic, while luciferase activity of EFNA3-MUT showed no significant difference (Figure 3 C). Meanwhile, RT-qPCR was conducted to test EFNA3 expression in HUVECs, and the experimental results revealed that miR-423-5p expression was raised in hucMSCs transfected with miR-423-5p mimic, while the expression of EFNA3 was reduced (Figure 3 D). This indicated that miR-423-5p was specifically bound to the EFNA3 gene.
Figure 3
Targeting relationship between miR-423-5p and EFNA3. A – Bioinformatics website TargetScan (https://www.targetscan.org/) predicted there were binding sites between EFNA3 and miR-423-5p; B – Binding sites between miR-423-5p and EFNA3; C – Results of dual luciferase reporter gene assay; D – miR-423-5p and EFNA3 mRNA expression levels in HUVECs were tested by RT-qPCR. *P < 0.05 vs. mimic NC group
miR-423-5p regulates EFNA3 to affect in vitro tube formation of HUVECs
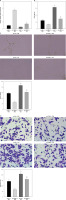
To further investigate whether hucMSC-EVs delivered miR-423-5p to affect in vitro tube formation of HUVECs by regulating EFNA3, we transfected pcDNA-EFNA3 into HUVECs and extracted hucMSC-EVs to treat HUVECs. The results of RT-qPCR revealed that EFNA3 expression levels were increased in HUVECs transfected with pcDNA-EFNA3. Furthermore, pcDNA-EFNA3 transfection could reverse the inhibiting effects of hucMSC-EVs on EFNA3 expression in HUVECs (Figure 4 A). Subsequently, the findings of pseudo-tube formation and Transwell assays demonstrated that pseudo-tube formation and migration capabilities of HUVECs were diminished in hucMSCs transfected with pcDNA-EFNA3, and pcDNA-EFNA3 reversed the promoting effects of hucMSC-EVs on HUVECs pseudo-tube formation and migration (Figures 4 B–D). The above results indicated that hucMSC-EV-miR-423-5p can promote HUVECs’ pseudo-tube formation and migration by inhibiting EFNA3.
Figure 4
Interaction effect of miR-423-5p and EFNA3 on in vitro tube formation of HUVECs. A – EFNA3 mRNA expression levels in HUVECs were assessed by RT-qPCR; B – HUVEC migration ability of each group was measured by Transwell assay. C – HUVEC angiogenic ability of each group was evaluated by pseudo-tube formation assay. D – VEGF content in cell supernatants was tested by ELISA. *P < 0.05 vs. the oe-NC + PBS group, #p < 0.05 vs. the oe-NC + EV group
EVs effectively protect cardiac function of MI rats in vivo by delivering miR-423-5p
To evaluate in vivo EVs’ effects on rat cardiac function, EVs were injected into MI rats. miR-423-5p and EFNA3 expression in myocardial tissues was tested by RT-qPCR, and the results showed that miR-423-5p expression was decreased while EFNA3 expression was increased in MI rats, and in the MI rats injected with EVs, miR-423-5p expression was elevated and EFNA3 expression was reduced. In addition, the trend was more pronounced in MI rats injected with EV-miR-423-5p (Figure 5 A). Furthermore, the cardiac function of rats in each group was evaluated, and it was found that there was a decrease in cardiac function as well as LVEF and LVFS values in MI rats, and there was a significant improvement in cardiac impairment and an increase in LVEF and LVFS values in MI rats injected with EVs, with a more pronounced trend in MI rats injected with EVs -miR-423-5p (Figure 5 B).
Figure 5
In vivo experiments for observing the effects of EV-miR-423-5p on cardiac function repair in MI rats. A – miR-423-5p in myocardial tissues was measured by RT-qPCR; B – LVEF and LVFS were determined by echocardiography to assess cardiac function. C – Myocardial fibrosis was evaluated by Masson staining. D – Positive capillary CD31 staining in the peri-infarct area was detected by immunohistochemistry. n = 10. E – Positive VEGF staining in the peri-infarct area was determined by immunohistochemistry. n = 10. *P < 0.05 vs the sham group, #p < 0.05 vs. the MI group, &p < 0.05 vs. the MI + EV-miR-NC group

To further explore in vivo hucMSC-EV miR-423-5p therapeutic potential in fibrosis and angiogenesis, Masson staining was conducted and the results showed a reduction in the area of fibrosis in MI rats injected with EVs (Figure 5 C). Capillaries were stained using immunohistochemistry with anti-CD31 and VEGF. After 2-week EV treatment of myocardial infarction, capillary density in the peri-infarct area was increased in MI rats injected with EVs (Figures 5 D, E). Additionally, this trend was more pronounced in MI rats injected with EV-miR-423-5p (Figures 5 D, E). EVs reduced the fibrosis area and promoted angiogenesis, thus significantly benefiting cardiac repair.
These results indicated that miR-423-5p secreted by hucMSC-EVs had a potential protective effect on cardiac function in infarcted rats.
Discussion
MI is a cardiovascular disorder with high mortality, and hucMSCs with forceful self-renewal capacity and multipotency offer the possibility of replacing damaged cardiomyocytes [6]. Advances have been achieved to identify the pathology and inhibit progression of the disease. Considering the delivery role of hucMSC-EVs, we revealed that hucMSC-EVs’ delivery of miR-423-5p alleviated MI in rats by suppressing EFNA3.
EVs are diverse, nanoscale membrane vesicles that are actively produced by cells [29]. As particles secreted by diverse cells, EVs are often observed to mimic their parent cell properties; as such, those originating from developmental sources hold promise in the treatment of a vast range of diseases including MI [30]. EVs originating from different cell types have potential to transport complicated information to endothelial cells and to induce pro- or antiangiogenic signaling [31]. In our study, we found that EVs reduced the fibrosis area and promoted angiogenesis, thus significantly benefiting cardiac repair. Similarly, in acute myocardial infarction (AMI), injured cardiac muscle cells release EVs with elevated levels of angiogenic, anti-apoptotic, mitogenic, and growth factors for the purpose of inducing infarcted myocardium repair and healing [32]. Meanwhile, MSC-EVs significantly improve cardiac function and angiogenesis in the post-MI heart. MSC-EVs increase the tube formation ability and proliferative and migratory ability of HUVECs [33].
miRNAs can modulate transcripts in situ and act as paracrine mediators in influencing angiogenesis at distant sites. miRNAs can shift cardiac proliferation, differentiation, maturation, as well as pathological remodeling responses to injury, stress, along with abnormal regulator expression [34]. Exosomal miRNAs are implicated in regulating endothelial cell function and angiogenesis [35]. It is supported that miR-423-5p has a relation to the prognosis of acute decompensated heart failure [36]. Early in acute MI, miR-423-5p expression in plasma is raised following normalization within 6 h, suggesting that miR-423-5p is a possible early marker of myocardial necrosis [37]. In our work, we evaluated the role of hucMSC-EVs, and finally noted that miR-423-5p could be enriched in hucMSC-EVs. Next, we evaluated the effects of miR-423-5p delivered by hucMSC-EVs in HUVECs, and eventually proved that hucMSC-EVs could promote in vitro tube formation by delivering miR-423-5p to HUVECs. Actually, it was previously demonstrated that the increase of miRNA sequences in the HepG2-secreted EVs indicates high levels of miR-423-5p [38]. There are articles focusing on the interactions of EVs with other miRNAs in MI. For instance, it was reported that delivery of miR-486-5p-engineered EVs safely promotes angiogenesis and heart function in a nonhuman primate MI model and may benefit cardiac repair [39]. Furthermore, in our study, in-vivo rat MI models were established, and hucMSC-EVs were injected into the MI rat heart infarcted area. Cardiac function, capillary density, and the degree of myocardial fibrosis were observed. The corresponding findings demonstrated that miR-423-5p secreted by hucMSC-EVs had a potential protective effect on cardiac function in infarcted rats.
The downstream gene involved in the functions of miR-432-5p secreted by hucMSC-EVs in MI was further analyzed. Previous studies have revealed that EFNA3 is linked to angiogenesis and EFNA3 downregulation promotes angiogenesis [17, 28]. At last, we validated the control of EFNA3 by miR-423-5p in MI. It was found that miR-423-5p was specifically bound to the EFNA3 gene, and was further demonstrated that hucMSC-EV-miR-423-5p could stimulate HUVECs’ pseudo-tube formation and migration by inhibiting EFNA3. MSC-EVs are sufficient to evoke angiogenesis and exert therapeutic effects on MI, and their pro-angiogenesis influence could be related to a miR-210- EFNA3 dependent mechanism [33]. Furthermore, small EVs derived from hypoxic MSCs evoke vascularized bone regeneration via the miR-210-3p/EFNA3/PI3K pathway [28].
To briefly conclude, our study showed that miR-423-5p delivered by hucMSC-EVs improves the cardiac function of MI rats and angiogenesis of HUVECs. Our study has deepened the understanding of the miR-423-5p/EFNA3 axis-modulated MI progression, and provided a basis for advancing potential therapeutics in MI. Our research is based on animal trials in the absence of clinical data, which is the major limitation of our analysis. Therefore, further research should be focused on clinical samples to validate the findings of our research. Additionally, in the in vitro experiments, we used only HUVEC as a model to investigate MI-induced angiogenesis. However, the mechanisms affecting MI are multifaceted, such as inflammatory response, oxidative stress, and apoptosis, which require further exploration in the follow-up work.








