SAPHO syndrome is a rare chronic autoinflammatory disease characterized by synovitis, acne, pustulosis, bone hypertrophy, and osteitis. While familial aggregation suggests a potential genetic basis, the precise underlying genetic mechanisms remain elusive. We identified a unique family cohort comprising four affected individuals, consisting of one male and three females. The pedigree diagram is shown in Figure 1. To our knowledge, this is the first reported instance of male patients within a familial context in the existing literature on SAPHO syndrome.
Fig. 1
Family diagram of patients with SAPHO syndrome
Boxes are male, circles are female; patients with SAPHO syndrome are shaded in gray; the diagonal line indicates death.
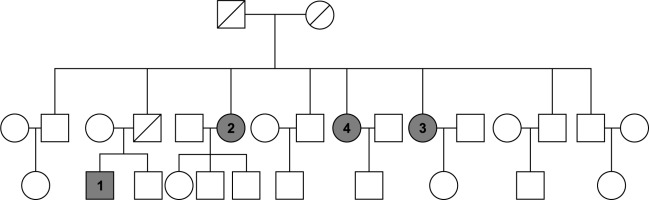
The primary patient was a male who first presented with palmar plantar pustulosis (PPP), followed by pain in the anterior chest wall and upper extremities. After a 99mTc-MDP whole-body bone scan, he was diagnosed with SAPHO syndrome. Subsequently, his three aunts also developed bone or skin symptoms, and after a 99mTc-MDP whole-body bone scan, they were all diagnosed with SAPHO syndrome. Among the four affected family members, all experienced anterior chest wall pain, with three displaying peripheral joint involvement and one of these exhibiting spinal manifestations. Additionally, three individuals presented with palmoplantar pustulosis, while one did not manifest any skin symptoms. However, in clinical observation, we found that skin lesions sometimes had long intervals between bone and joint manifestations, so the possibility of later development of hand and foot pustules in this patient cannot be ruled out. The chronological onset and clinical details of the familial cases are outlined in Table 1 (in order of onset).
Table 1
Case data of patients of this family
SAPHO syndrome typically presents in adulthood, exhibiting a higher prevalence among females. Manifestations primarily involve the bone and joints, with or without concurrent skin lesions, representing a hallmark of the disease. The pathogenesis of SAPHO syndrome remains elusive, likely being associated with factors such as infection, immune dysregulation, and genetics. Clinical diversity often leads to misdiagnosis as bone and joint disorders or dermatological conditions.
Previous reports have highlighted familial aggregation of SAPHO cases [1-3], particularly among first-degree relatives. A recent large cohort study identified significant familial clustering of SAPHO syndrome in Chinese female patients, with mother-daughter and sister-sister pairs being the most commonly observed relationships [4]. Genetic associations with SAPHO syndrome have also been reported in male patients, albeit with insufficient osteoarticular evaluation. The family chart of our patient’s disease suggests that SAPHO syndrome may be a polygenic disorder, given its occurrence in multiple family members of different genders (aunts and nephews) across generations, diverging from the inheritance patterns of monogenic disorders.
Based on investigations into several families, researchers have conducted studies on genetic factors in SAPHO syndrome. Guo et al. [5] found a strong correlation between SAPHO syndrome and specific genetic variants, including IL-4 rs2243248, IL-23R rs10889677, rs2201841, and rs7517847. Individuals harboring the A-G-C-G-T haplotype of IL-23 were shown to have an increased susceptibility to SAPHO syndrome. Assmann et al. [6] identified correlations between SAPHO syndrome and genetic markers such as p53 G72C, Mdm2 T309G, as well as copy number variations in CSF2RA and NOD2, and copy number amplifications in MEGF6 and ADAM 5, all implicated in the pathogenesis of SAPHO syndrome [7]. While sharing some pathological, clinical, and radiographic features with spondyloarthritis, subsequent investigations have discounted the involvement of HLA-B27, HLA-Cw6, and HLA-DR in the development of SAPHO syndrome [8].
In conclusion, compelling evidence suggests that genetic predispositions may significantly contribute to the etiology of SAPHO syndrome, a complex and multifaceted condition characterized by a spectrum of clinical manifestations. Despite the intriguing possibility of genetic involvement, the current report lacks comprehensive genetic sequencing data for the familial cases under investigation. Future endeavors should prioritize genetic sequencing and other molecular genetic analyses to elucidate the underlying genetic architecture. Such studies could potentially uncover novel pathogenic genes, thereby enhancing our comprehension of the intricate disease mechanisms. Moreover, these insights could pave the way for the development of targeted therapies and personalized treatment strategies, ultimately improving the clinical management and prognosis of individuals afflicted with SAPHO syndrome.


